Targeting Glioma Stem Cell-Derived Pericytes Disrupts the Blood-Tumor Barrier and Improves Chemotherapeutic Efficacy
- PMID: 29100012
- PMCID: PMC5687837
- DOI: 10.1016/j.stem.2017.10.002
Targeting Glioma Stem Cell-Derived Pericytes Disrupts the Blood-Tumor Barrier and Improves Chemotherapeutic Efficacy
Abstract
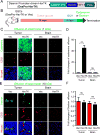
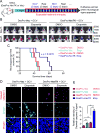
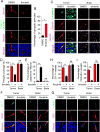
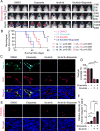
The blood-tumor barrier (BTB) is a major obstacle for drug delivery to malignant brain tumors such as glioblastoma (GBM). Disrupting the BTB is therefore highly desirable but complicated by the need to maintain the normal blood-brain barrier (BBB). Here we show that targeting glioma stem cell (GSC)-derived pericytes specifically disrupts the BTB and enhances drug effusion into brain tumors. We found that pericyte coverage of tumor vasculature is inversely correlated with GBM patient survival after chemotherapy. Eliminating GSC-derived pericytes in xenograft models disrupted BTB tight junctions and increased vascular permeability. We identified BMX as an essential factor for maintaining GSC-derived pericytes. Inhibiting BMX with ibrutinib selectively targeted neoplastic pericytes and disrupted the BTB, but not the BBB, thereby increasing drug effusion into established tumors and enhancing the chemotherapeutic efficacy of drugs with poor BTB penetration. These findings highlight the clinical potential of targeting neoplastic pericytes to significantly improve treatment of brain tumors.
Keywords: BMX; blood-brain barrier (BBB); blood-tumor barrier (BTB); glioma stem cells; ibrutinib; pericytes.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA. Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res. 2006;66:9054–9064. - PubMed
-
- Alifrangis C, Agarwal R, Short D, Fisher RA, Sebire NJ, Harvey R, Savage PM, Seckl MJ. EMA/CO for high-risk gestational trophoblastic neoplasia: good outcomes with induction low-dose etoposide-cisplatin and genetic analysis. J Clin Oncol. 2013;31:280–286. - PubMed
-
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. - PubMed
-
- Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, He L, Norlin J, Lindblom P, Strittmatter K, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

