ANGPTL4 promotes the progression of cutaneous melanoma to brain metastasis
- PMID: 29100268
- PMCID: PMC5652662
- DOI: 10.18632/oncotarget.19018
ANGPTL4 promotes the progression of cutaneous melanoma to brain metastasis
Abstract
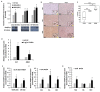
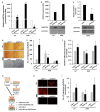
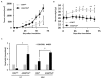
In an ongoing effort to identify molecular determinants regulating melanoma brain metastasis, we previously identified Angiopoietin-like 4 (ANGPTL4) as a component of the molecular signature of such metastases. The aim of this study was to determine the functional significance of ANGPTL4 in the shaping of melanoma malignancy phenotype, especially in the establishment of brain metastasis. We confirmed that ANGPTL4 expression is significantly higher in cells metastasizing to the brain than in cells from the cutaneous (local) tumor from the same melanoma in a nude mouse xenograft model, and also in paired clinical specimens of melanoma metastases than in primary melanomas from the same patients. In vitro experiments indicated that brain-derived soluble factors and transforming growth factor β1 (TGFβ1) up-regulated ANGPTL4 expression by melanoma cells. Forced over-expression of ANGPTL4 in cutaneous melanoma cells promoted their ability to adhere and transmigrate brain endothelial cells. Over-expressing ANGPTL4 in cells derived from brain metastases resulted in the opposite effects. In vivo data indicated that forced overexpression of ANGPTL4 promoted the tumorigenicity of cutaneous melanoma cells but did not increase their ability to form brain metastasis. This finding can be explained by inhibitory activities of brain-derived soluble factors. Taken together these findings indicate that ANGPTL4 promotes the malignancy phenotype of primary melanomas of risk to metastasize to the brain.
Keywords: ANGPTL4; TGFβ1; brain; melanoma; metastasis.
Conflict of interest statement
CONFLICTS OF INTEREST The Authors do not have any conflicts of interest.
Figures



Similar articles
-
The metastatic microenvironment: Brain-derived soluble factors alter the malignant phenotype of cutaneous and brain-metastasizing melanoma cells.Int J Cancer. 2012 Dec 1;131(11):2509-18. doi: 10.1002/ijc.27552. Epub 2012 Apr 12. Int J Cancer. 2012. PMID: 22447293
-
The metastatic microenvironment: Claudin-1 suppresses the malignant phenotype of melanoma brain metastasis.Int J Cancer. 2015 Mar 15;136(6):1296-307. doi: 10.1002/ijc.29090. Epub 2014 Sep 8. Int J Cancer. 2015. PMID: 25046141
-
CCR4 is a determinant of melanoma brain metastasis.Oncotarget. 2017 May 9;8(19):31079-31091. doi: 10.18632/oncotarget.16076. Oncotarget. 2017. PMID: 28415693 Free PMC article.
-
Hypoxia-inducible factor 1 alpha-activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin β1 signaling in human hepatocellular carcinoma.Hepatology. 2011 Sep 2;54(3):910-9. doi: 10.1002/hep.24479. Hepatology. 2011. PMID: 21674552
-
Interaction of tumor cells and astrocytes promotes breast cancer brain metastases through TGF-β2/ANGPTL4 axes.NPJ Precis Oncol. 2019 Oct 3;3:24. doi: 10.1038/s41698-019-0094-1. eCollection 2019. NPJ Precis Oncol. 2019. PMID: 31602400 Free PMC article.
Cited by
-
The Challenge of Classifying Metastatic Cell Properties by Molecular Profiling Exemplified with Cutaneous Melanoma Cells and Their Cerebral Metastasis from Patient Derived Mouse Xenografts.Mol Cell Proteomics. 2020 Mar;19(3):478-489. doi: 10.1074/mcp.RA119.001886. Epub 2019 Dec 31. Mol Cell Proteomics. 2020. PMID: 31892524 Free PMC article.
-
Upregulation of cell surface GD3 ganglioside phenotype is associated with human melanoma brain metastasis.Mol Oncol. 2020 Aug;14(8):1760-1778. doi: 10.1002/1878-0261.12702. Epub 2020 Jun 5. Mol Oncol. 2020. PMID: 32358995 Free PMC article.
-
Site-specific metastasis: A cooperation between cancer cells and the metastatic microenvironment.Int J Cancer. 2021 Mar 15;148(6):1308-1322. doi: 10.1002/ijc.33247. Epub 2020 Aug 27. Int J Cancer. 2021. PMID: 32761606 Free PMC article. Review.
-
Cystatin C takes part in melanoma-microglia cross-talk: possible implications for brain metastasis.Clin Exp Metastasis. 2018 Aug;35(5-6):369-378. doi: 10.1007/s10585-018-9891-0. Epub 2018 May 2. Clin Exp Metastasis. 2018. PMID: 29722001 Free PMC article.
-
Emerging roles of angiopoietin‑like 4 in human tumors (Review).Int J Oncol. 2025 Feb;66(2):9. doi: 10.3892/ijo.2024.5715. Epub 2024 Dec 20. Int J Oncol. 2025. PMID: 39704206 Free PMC article. Review.
References
-
- Marzese DM, Witz IP, Kelly DF, Hoon DS. Epigenomic landscape of melanoma progression to brain metastasis: unexplored therapeutic alternatives. Epigenomics. 2015;7:1303–11. - PubMed
-
- Bafaloukos D, Gogas H. The treatment of brain metastases in melanoma patients. Cancer Treat Rev. 2004;30:515–20. - PubMed
-
- Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, Davis DW, McConkey DJ, Fidler IJ. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res. 2000;60:4959–67. - PubMed
-
- Klein A, Schwartz H, Sagi-Assif O, Meshel T, Izraely S, Ben Menachem S, Bengaiev R, Ben-Shmuel A, Nahmias C, Couraud PO, Witz IP, Erez N. Astrocytes facilitate melanoma brain metastasis via secretion of IL-23. J Pathol. 2015;236:116–27. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

