Nicotine induces EP4 receptor expression in lung carcinoma cells by acting on AP-2α: The intersection between cholinergic and prostanoid signaling
- PMID: 29100274
- PMCID: PMC5652668
- DOI: 10.18632/oncotarget.18023
Nicotine induces EP4 receptor expression in lung carcinoma cells by acting on AP-2α: The intersection between cholinergic and prostanoid signaling
Abstract
It was demonstrated that nicotine increased non-small cell lung cancer cell proliferation through nicotinic acetylcholine receptor -mediated signals. However, the detailed mechanism remains incompletely understood. We evaluated whether nicotine increased EP4 receptor expression in lung carcinoma cells by activating on AP-2α.
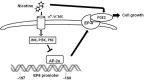
Methods: The non-small cell lung cancer cells of A549 and H1838 were cultured and treated with EP4 inhibitor AH23848, also with EP4 and control siRNAs. The extracellular signal-regulated kinases inhibitor PD98059, the p38 mitogen-activated protein kinase inhibitor SB239063, the α7 nicotinic acetylcholine receptor inhibitor α-bungarotoxin, the α4 nicotinic acetylcholine receptor inhibitor dihydro-β-erythroidine, the PI3K inhibitor wortmannin, the PKC inhibitor calphostin C, and the PKA inhibitor H89 have been used to evaluate the effects on proliferations. It indicates that nicotine increases EP4 expression through α7 nicotinic acetylcholine receptor-dependent activations of PI3-K, JNK and PKC pathways that leads to reduction of AP-2α-DNA binding. This, together with the elevated secretion of PGE2, further enhances the tumor promoting effects of nicotine. These studies suggest a novel molecular mechanism by which nicotine increases non-small cell lung cancer cell proliferation.
Keywords: acetylcholine receptor; cyclooxygenases-2; nicotine; non-small cell lung carcinoma; proliferation.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare that they have no competing interests.
Figures




Similar articles
-
Nicotine stimulates human lung cancer cell growth by inducing fibronectin expression.Am J Respir Cell Mol Biol. 2007 Dec;37(6):681-90. doi: 10.1165/rcmb.2007-0051OC. Epub 2007 Jun 28. Am J Respir Cell Mol Biol. 2007. PMID: 17600315
-
Nicotine stimulates PPARbeta/delta expression in human lung carcinoma cells through activation of PI3K/mTOR and suppression of AP-2alpha.Cancer Res. 2009 Aug 15;69(16):6445-53. doi: 10.1158/0008-5472.CAN-09-1001. Epub 2009 Aug 4. Cancer Res. 2009. PMID: 19654299 Free PMC article.
-
Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells.Pulm Pharmacol Ther. 2007;20(6):629-41. doi: 10.1016/j.pupt.2006.07.001. Epub 2006 Aug 18. Pulm Pharmacol Ther. 2007. PMID: 17015027
-
Novel link between prostaglandin E2 (PGE2) and cholinergic signaling in lung cancer: The role of c-Jun in PGE2-induced α7 nicotinic acetylcholine receptor expression and tumor cell proliferation.Thorac Cancer. 2015 Jul;6(4):488-500. doi: 10.1111/1759-7714.12219. Epub 2015 Jan 14. Thorac Cancer. 2015. PMID: 26273406 Free PMC article.
-
Nicotine-Mediated Regulation of Nicotinic Acetylcholine Receptors in Non-Small Cell Lung Adenocarcinoma by E2F1 and STAT1 Transcription Factors.PLoS One. 2016 May 26;11(5):e0156451. doi: 10.1371/journal.pone.0156451. eCollection 2016. PLoS One. 2016. PMID: 27228072 Free PMC article.
Cited by
-
Acetylcholine signaling system in progression of lung cancers.Pharmacol Ther. 2019 Feb;194:222-254. doi: 10.1016/j.pharmthera.2018.10.002. Epub 2018 Oct 3. Pharmacol Ther. 2019. PMID: 30291908 Free PMC article. Review.
-
Nicotinic receptors in airway disease.Am J Physiol Lung Cell Mol Physiol. 2024 Feb 1;326(2):L149-L163. doi: 10.1152/ajplung.00268.2023. Epub 2023 Dec 12. Am J Physiol Lung Cell Mol Physiol. 2024. PMID: 38084408 Free PMC article. Review.
-
Celecoxib prevents malignant progression of smoking-induced lung tumors via suppression of the COX-2/PGE2 signaling pathway in mice.Front Immunol. 2025 Mar 19;16:1557790. doi: 10.3389/fimmu.2025.1557790. eCollection 2025. Front Immunol. 2025. PMID: 40176805 Free PMC article.
-
Nicotine promotes the progression and metastasis of non-small cell lung cancer by modulating the OTUB1-c-Myc-EZH2 axis.Acta Pharmacol Sin. 2025 Sep;46(9):2509-2521. doi: 10.1038/s41401-025-01527-5. Epub 2025 Apr 1. Acta Pharmacol Sin. 2025. PMID: 40169782
-
Cell signaling and epigenetic regulation of nicotine-induced carcinogenesis.Environ Pollut. 2024 Mar 15;345:123426. doi: 10.1016/j.envpol.2024.123426. Epub 2024 Jan 29. Environ Pollut. 2024. PMID: 38295934 Free PMC article. Review.
References
-
- Mediber K, Freidja ML, Grelet S, Lorenzato M, Maouche K, Nawrocki-Raby B, Birembaut P, Polette M, Tournier JM. Role of nicotinic acetylcholine receptors in cell proliferation and tumour invasion in broncho-pulmonary carcinomas. Lung cancer. 2015;87:258–264. - PubMed
-
- Pillai S, Chellappan S. alpha7 nicotinic acetylcholine receptor subunit in angiogenesis and epithelial to mesenchymal transition. Current drug targets. 2012;13:671–679. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

