Chemosensitivity-directed therapy compared to dacarbazine in chemo-naive advanced metastatic melanoma: a multicenter randomized phase-3 DeCOG trial
- PMID: 29100289
- PMCID: PMC5652683
- DOI: 10.18632/oncotarget.18635
Chemosensitivity-directed therapy compared to dacarbazine in chemo-naive advanced metastatic melanoma: a multicenter randomized phase-3 DeCOG trial
Abstract
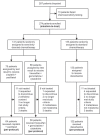
Chemotherapy still plays an important role in metastatic melanoma, particularly for patients who are not suitable or have no access to highly efficacious new therapies. Pre-therapeutic chemosensitivity testing might be useful to identify optimal chemotherapy regimens for individual patients. This multicenter randomized phase-3 trial was aimed to test for superiority of chemosensitivity-directed combination chemotherapy compared to standard dacarbazine monochemotherapy, and to demonstrate the chemosensitivity test result as prognostic in metastatic melanoma. Chemo-naive patients with advanced melanoma were biopsied from metastatic lesions. Tumor cells were isolated and tested ex-vivo for sensitivity to chemotherapeutic agents using an ATP-based viability assay. Patients with evaluable test results were randomly assigned to receive either chemosensitivity-directed combination chemotherapy (paclitaxel+cisplatin, treosulfan+gemcitabine, treosulfan+cytarabine), or dacarbazine. The primary study endpoint was overall survival (OS). After inclusion of 287 patients and a median follow-up of 26 months, the per-protocol population (n=244) showed no difference in OS between chemosensitivity-directed therapy and dacarbazine (median 9.2 vs 9.0 months, HR=1.08, p=0.64). The disease control rate (CR+PR+SD) tended to be higher in patients treated with chemosensitivity-directed therapy (32.8% vs 23.0%, p=0.088); objective response rates (CR+PR) showed no difference between groups (10.7% vs 12.3%, p=0.90). Patients whose tumors were tested chemosensitive showed no better OS or response rate than patients with chemoresistant tumors. Severe toxicities (CTC grade 3-4) were significantly more frequently observed with chemosensitivity-directed combination chemotherapy than with dacarbazine (40.2% vs 12.3%, p<0.0001). These results indicate, that chemosensitivity-directed combination chemotherapy is not superior to dacarbazine, but leads to significantly more severe toxicities.
Keywords: chemosensitivity; individualized chemotherapy; melanoma; phase-3 trial.
Conflict of interest statement
CONFLICTS OF INTEREST This is an investigator-initiated trial without financial support from pharmaceutical companies. The authors declare no potential conflicts of interest concerning the pharmaceutical agents used in this trial. Additional conflicts of interest: Selma Ugurel declares research support from medac and BMS, speaker's and advisory board honoraria from BMS, MSD, and Roche, and travel support from BMS, medac, MSD, and Roche. Carmen Loquai declares advisory board honoraria from Roche, Novartis, Pierre Fabre, BMS, MSD, Leo and Amgen, travel reimbursement from Roche, Novartis, BMS, and Amgen, and speakers honoraria from Roche, Novartis, BMS, MSD, and Amgen. Patrick Terheyden declares speakers honoraria from BMS, Novartis, and Roche, consultant´s honoraria from BMS, Merck, Novartis, and Roche, and travel support from BMS and Roche. Dirk Schadendorf declares research support from Roche, Novartis, Amgen, BMS, and Merck, honoraria for lectures from Roche, Novartis, MSD, BMS, and Amgen, and honoraria for advisory boards from Roche, Novartis, BMS, Merck, and Amgen. Jochen Utikal declares speakers and advisory board honoraria and travel support from Amgen, BMS, GSK, Leo, MSD, Novartis, and Roche. Ralf Gutzmer declares research support from Roche, Novartis, Pfizer, and Johnson&Johnson, honoraria for lectures from Roche, BMS, GSK, Novartis, Merck Serono, MSD, Almirall-Hermal, Amgen, Galderma, Janssen, and Boehringer Ingelheim, honoraria for advisory boards from Roche, BMS, GSK, Novartis, MSD, Almirall-Hermal, LEO, Amgen, Pfizer, and Pierre-Fabre, and travel support from Roche and BMS. Katharina Kähler declares consultant fees from Roche, BMS, and MSD, travel grants and speaker fees from Roche, BMS, MSD, Novartis, and Amgen. Rudolf Stadler declares advisory honoraria from Takeda, Roche, and Actelion. Christoph Höller declares speakers and advisory board honoraria from Astra Zeneca, Amgen, BMS, MSD, Novartis, Pierre-Fabre, and Roche. Jürgen C. Becker discloses grant support and/or advisory board honoraria from Amgen, BMS, CureVac, Lytex, Merck Serono, Novartis, Pfizer, Rigontec, Takeda and Roche.
Figures


References
-
- Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, Moon J, Sondak VK, Atkins MB, Eisenhauer EA, Parulekar W, Markovic SN, Saxman S, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26:527–534. - PubMed
-
- Eigentler TK, Caroli UM, Radny P, Garbe C. Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol. 2003;4:748–759. - PubMed
-
- Ugurel S, Röhmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, Larkin J, Long GV, Lorigan P, McArthur GA, Ribas A, Robert C, Schadendorf D, et al. Survival of patients with advanced metastatic melanoma: The impact of novel therapies. Eur J Cancer. 2016;53:125–34. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

