KRT8 upregulation promotes tumor metastasis and is predictive of a poor prognosis in clear cell renal cell carcinoma
- PMID: 29100303
- PMCID: PMC5652697
- DOI: 10.18632/oncotarget.19198
KRT8 upregulation promotes tumor metastasis and is predictive of a poor prognosis in clear cell renal cell carcinoma
Abstract
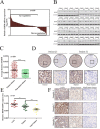
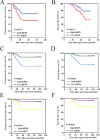
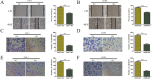
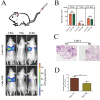
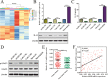
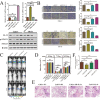
Keratin 8 (KRT8) plays an essential role in the development and metastasis of multiple human cancers. However, its role in clear cell renal cell carcinoma (ccRCC) remains unexplored. Here, we investigated the expression pattern, clinical significance, and function of KRT8 in ccRCC. KRT8 mRNA and protein levels were determined in two large cohorts using quantitative real-time polymerase chain reaction (qRT-PCR) and tissue microarray (TMA) immunohistochemistry (IHC), respectively. We found that KRT8 expression was upregulated in ccRCC and vein tumor thrombi (VTTs). KRT8 overexpression in ccRCC was significantly correlated with aggressive characteristics and was predictive of a poor prognosis in ccRCC patients. Moreover, KRT8 overexpression in renal cancer cell lines promoted cell migration and invasion. In contrast, KRT8 knockdown suppressed ccRCC metastasis both in vitro and in vivo. In addition, our findings showed that KRT8 promoted ccRCC metastasis by increasing IL-11 expression, causing IL-11 autocrine induction, and triggering STAT3 signaling. Overall, this study established the significance of KRT8-IL-11 axis activation in aggressive ccRCC and defined a novel critical signaling mechanism that drives human ccRCC invasion and metastasis.
Keywords: IL-11; KRT8; biomarker; clear cell renal cell carcinoma; metastasis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors disclose no conflicts of interest.
Figures
Similar articles
-
Hypoxia-induced overexpression of stanniocalcin-1 is associated with the metastasis of early stage clear cell renal cell carcinoma.J Transl Med. 2015 Feb 12;13:56. doi: 10.1186/s12967-015-0421-4. J Transl Med. 2015. PMID: 25740019 Free PMC article.
-
Downregulation of FOXO3a promotes tumor metastasis and is associated with metastasis-free survival of patients with clear cell renal cell carcinoma.Clin Cancer Res. 2014 Apr 1;20(7):1779-90. doi: 10.1158/1078-0432.CCR-13-1687. Epub 2014 Jan 31. Clin Cancer Res. 2014. PMID: 24486593
-
Long noncoding RNA MRCCAT1 promotes metastasis of clear cell renal cell carcinoma via inhibiting NPR3 and activating p38-MAPK signaling.Mol Cancer. 2017 Jun 28;16(1):111. doi: 10.1186/s12943-017-0681-0. Mol Cancer. 2017. PMID: 28659173 Free PMC article.
-
Up-regulation of SR-BI promotes progression and serves as a prognostic biomarker in clear cell renal cell carcinoma.BMC Cancer. 2018 Jan 22;18(1):88. doi: 10.1186/s12885-017-3761-z. BMC Cancer. 2018. PMID: 29357836 Free PMC article.
-
Emerging insights into keratin 7 roles in tumor progression and metastasis of cancers.Front Oncol. 2024 Jan 8;13:1243871. doi: 10.3389/fonc.2023.1243871. eCollection 2023. Front Oncol. 2024. PMID: 38260844 Free PMC article. Review.
Cited by
-
SurviveAI: Long Term Survival Prediction of Cancer Patients Based on Somatic RNA-Seq Expression.Cancer Inform. 2022 Oct 7;21:11769351221127875. doi: 10.1177/11769351221127875. eCollection 2022. Cancer Inform. 2022. PMID: 36225330 Free PMC article. Review.
-
KRT8 Serves as a Novel Biomarker for LUAD and Promotes Metastasis and EMT via NF-κB Signaling.Front Oncol. 2022 May 19;12:875146. doi: 10.3389/fonc.2022.875146. eCollection 2022. Front Oncol. 2022. PMID: 35664775 Free PMC article.
-
Transcriptomic data in tumor-adjacent normal tissues harbor prognostic information on multiple cancer types.Cancer Med. 2023 May;12(10):11960-11970. doi: 10.1002/cam4.5864. Epub 2023 Mar 31. Cancer Med. 2023. PMID: 36999961 Free PMC article.
-
Cytoskeletal Remodeling in Cancer.Biology (Basel). 2020 Nov 7;9(11):385. doi: 10.3390/biology9110385. Biology (Basel). 2020. PMID: 33171868 Free PMC article. Review.
-
ZNF384 mediates KRT23 to promote CRC process through the TGF-β/Smad signaling pathway.Cytotechnology. 2025 Jun;77(3):111. doi: 10.1007/s10616-025-00765-z. Epub 2025 May 30. Cytotechnology. 2025. PMID: 40453923
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373:1119–1132. - PubMed
-
- Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L, Merseburger AS, Mulders P, Powles T, Staehler M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67:913–924. - PubMed
-
- Novara G, Ficarra V, Antonelli A, Artibani W, Bertini R, Carini M, Cosciani CS, Imbimbo C, Longo N, Martignoni G, Martorana G, Minervini A, Mirone V, et al. Validation of the 2009 TNM version in a large multi-institutional cohort of patients treated for renal cell carcinoma: are further improvements needed. Eur Urol. 2010;58:588–595. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

