Role of DNA methylation in perinatal nicotine-induced development of heart ischemia-sensitive phenotype in rat offspring
- PMID: 29100355
- PMCID: PMC5652749
- DOI: 10.18632/oncotarget.20172
Role of DNA methylation in perinatal nicotine-induced development of heart ischemia-sensitive phenotype in rat offspring
Abstract
Background and purpose: Maternal cigarette smoking increases the risk of cardiovascular disease in offspring. Recently, we have demonstrated that perinatal nicotine exposure alters heart development and increases heart susceptibility to ischemia/reperfusion (I/R) injury in rat offspring. The present study tested the hypothesis that DNA methylation plays a key role in the nicotine-induced development of heart ischemia-sensitive phenotype in offspring.
Experimental approach: Nicotine was administered to pregnant rats via subcutaneous osmotic minipumps from gestational day 4 until postnatal day 10. After birth, the postnatal offspring were treated with the DNA methylation inhibitor, 5-aza-2'-deoxycytidine (5-Aza) or saline from postnatal day 3 to day 10. Experiments were conducted in 1 month old offspring.
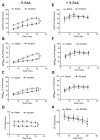
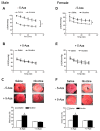
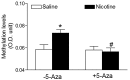
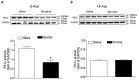
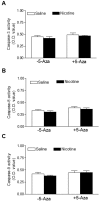
Key results: Perinatal nicotine increased I/R-induced left ventricular (LV) injury, and decreased post-ischemic recovery of the LV function and coronary flow rate in both male and female offspring. Nicotine differentially increased DNMT3a expression and global DNA methylation levels in LV tissues. Treatment with 5-Aza inhibited nicotine-induced an increase in DNMT3a and global DNA methylation, and blocked the nicotine-induced increase in I/R injury and dysfunction in the heart. In addition, nicotine attenuated protein kinases Cε and large-conductance Ca(2+)-activated K(+) (BKca) channel β1 subunit protein abundances in the heart, which were reversed by 5-Aza treatment.
Conclusions and implications: The present findings provide novel evidence that the increased DNA methylation plays a causal role in nicotine-induced development of heart ischemic sensitive phenotype, and suggest a potential therapeutic target of DNA demethylation for the fetal programming of heart ischemic disease later in life.
Keywords: DNA methylation; heart ischemia-sensitive phenotype; perinatal nicotine.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures



References
-
- Beratis NG, Panagoulias D, Varvarigou A. Increased blood pressure in neonates and infants whose mothers smoked during pregnancy. The Journal of pediatrics. 1996;128:806–812. - PubMed
-
- Blake KV, Gurrin LC, Evans SF, Beilin LJ, Landau LI, Stanley FJ, et al. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early human development. 2000;57:137–147. - PubMed
-
- Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;1998;285:931–945. - PubMed
-
- Pausová Z, Paus T, Sedová L, Bérubé J. Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats: a case of gene-environment interaction. Kidney Int. 2003;64:829–835. - PubMed
-
- Xiao D, Huang X, Lawrence J, Yang S, Zhang L. Fetal and neonatal nicotine exposure differentially regulates vascular contractility in adult male and female offspring. J Pharmacol Exp Ther. 2007;320:654–661. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

