Metabolic modulation of Ewing sarcoma cells inhibits tumor growth and stem cell properties
- PMID: 29100387
- PMCID: PMC5652780
- DOI: 10.18632/oncotarget.20467
Metabolic modulation of Ewing sarcoma cells inhibits tumor growth and stem cell properties
Abstract
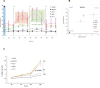
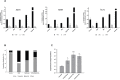
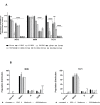
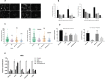
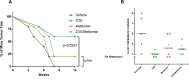
Ewing sarcoma (EWS) is a highly aggressive and metabolically active malignant tumor. Metabolic activity can broadly be characterized by features of glycolytic activity and oxidative phosphorylation. We have further characterized metabolic features of EWS cells to identify potential therapeutic targets. EWS cells had significantly more glycolytic activity compared to their non-malignant counterparts. Thus, metabolic inhibitors of glycolysis such as 2-deoxy-D-glucose (2DG) and of the mitochondrial respiratory pathway, such as metformin, were evaluated as potential therapeutic agents against a panel of EWS cell lines in vitro. Results indicate that 2DG alone or in combination with metformin was effective at inducing cell death in EWS cell lines. The predominant mechanism of cell death appears to be through stimulating apoptosis leading into necrosis with concomitant activation of AMPK-α. Furthermore, we demonstrate that the use of metabolic modulators can target putative EWS stem cells, both in vitro and in vivo, and potentially overcome chemotherapeutic resistance in EWS. Based on these data, clinical strategies using drugs targeting tumor cell metabolism present a viable therapeutic modality against EWS.
Keywords: 2DG; Ewing sarcoma; cancer stem cells; metabolism; metformin.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no competing financial interest.
Figures


References
-
- Riggi N, Stamenkovic I. The biology of Ewing sarcoma. Cancer Lett. 2007;254:1–10. - PubMed
-
- Balamuth NJ, Womer RB. Ewing's sarcoma. Lancet Oncol. 2010;11:184–192. - PubMed
-
- Esiashvili N, Goodman M, Marcus RB., Jr Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. J Pediatr Hemat Oncol. 2008;30:425–430. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

