Meta-analysis of the mutational status of circulation tumor cells and paired primary tumor tissues from colorectal cancer patients
- PMID: 29100436
- PMCID: PMC5652825
- DOI: 10.18632/oncotarget.18272
Meta-analysis of the mutational status of circulation tumor cells and paired primary tumor tissues from colorectal cancer patients
Abstract
As predictive markers for anti-EGFR therapy, KRAS and BRAF mutations are routinely detected in primary and metastatic colorectal cancer (CRC) cells, but seldom in circulating tumor cells (CTCs). Detecting mutations in CTCs could help explain mutational differences between tumor cells at local sites and distant metastases, thereby improving treatment outcomes. Here, we conducted a systematic review and meta-analysis to compare KRAS and BRAF mutations in paired CTCs and primary tumors from CRC patients, to detect any possible discordance. A total of 244 CRC patients from nine studies were included. Our subgroup meta-analysis demonstrated that the total odds ratio for mutations in CTCs was only 55% of that in primary tumors in the stage IV subgroup. We also found low heterogeneity among studies and differences in mutations between CTCs and primary tumors in the stage IV subgroup (I2 = 0%, P = 0.01). We observed a higher frequency of KRAS mutations in CTCs than in primary tumors at early stages (I + II), a similar frequency in stage III, and a lower frequency in stage IV. There were also differences among the Epcam-targeted CTC enrichment, PCR-based mutation profiling, and ≥ 3 CTCs enriched (I2 = 0%, P = 0.03) subgroups. These finding indicate mutational discordance between CTCs and primary CRCs, particularly in the stage IV and KRAS subgroups. We suggest large-sample studies stratified by clinical stage and KRAS subtype are urgently warranted to accurately evaluate mutational variations in CTCs compared to primary and metastatic CRC cells.
Keywords: BRAF mutation; KRAS mutation; circulating tumor cells; clinical stage; colorectal cancer.
Conflict of interest statement
CONFLICTS OF INTEREST None to declare.
Figures
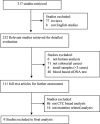







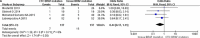

Similar articles
-
KRAS and BRAF mutation status in circulating colorectal tumor cells and their correlation with primary and metastatic tumor tissue.Int J Cancer. 2013 Jul;133(1):130-41. doi: 10.1002/ijc.27987. Epub 2013 Feb 9. Int J Cancer. 2013. PMID: 23233388
-
Mutational analysis of circulating tumor cells from colorectal cancer patients and correlation with primary tumor tissue.PLoS One. 2015 Apr 22;10(4):e0123902. doi: 10.1371/journal.pone.0123902. eCollection 2015. PLoS One. 2015. PMID: 25902072 Free PMC article.
-
Comparison of neuroendocrine differentiation and KRAS/NRAS/BRAF/PIK3CA/TP53 mutation status in primary and metastatic colorectal cancer.Int J Clin Exp Pathol. 2014 Aug 15;7(9):5927-39. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25337237 Free PMC article.
-
Clinical Implications and Future Perspectives of Circulating Tumor Cells and Biomarkers in Clinical Outcomes of Colorectal Cancer.Transl Oncol. 2016 Aug;9(4):340-7. doi: 10.1016/j.tranon.2016.06.006. Transl Oncol. 2016. PMID: 27567958 Free PMC article. Review.
-
Circulating tumor cells in colorectal cancer - a review of detection methods and clinical relevance.Contemp Oncol (Pozn). 2023;27(3):123-131. doi: 10.5114/wo.2023.133740. Epub 2023 Dec 21. Contemp Oncol (Pozn). 2023. PMID: 38239860 Free PMC article. Review.
Cited by
-
Distinct mutation profiles between primary bladder cancer and circulating tumor cells warrant the use of circulating tumors cells as cellular resource for mutation follow-up.BMC Cancer. 2020 Dec 7;20(1):1203. doi: 10.1186/s12885-020-07684-6. BMC Cancer. 2020. PMID: 33287735 Free PMC article.
-
Circulating tumor cell profiling for precision oncology.Mol Oncol. 2021 Jun;15(6):1622-1646. doi: 10.1002/1878-0261.12901. Epub 2021 Feb 1. Mol Oncol. 2021. PMID: 33448107 Free PMC article. Review.
-
Prospective Monitoring of Circulating Epithelial Tumor Cells (CETC) Reveals Changes in Gene Expression during Adjuvant Radiotherapy of Breast Cancer Patients.Curr Oncol. 2021 Sep 8;28(5):3507-3524. doi: 10.3390/curroncol28050302. Curr Oncol. 2021. PMID: 34590615 Free PMC article.
-
PIK3CA hotspot mutations in circulating tumor cells and paired circulating tumor DNA in breast cancer: a direct comparison study.Mol Oncol. 2019 Dec;13(12):2515-2530. doi: 10.1002/1878-0261.12540. Epub 2019 Sep 30. Mol Oncol. 2019. PMID: 31254443 Free PMC article.
-
Circulating Tumor Cells as a Tool for Assessing Tumor Heterogeneity.Theranostics. 2019 Jun 19;9(16):4580-4594. doi: 10.7150/thno.34337. eCollection 2019. Theranostics. 2019. PMID: 31367241 Free PMC article. Review.
References
-
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–17. - PubMed
-
- Karapetis CS, Khambata-Ford S, Jonker DJ, O’Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359:1757–65. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

