Inhibition of PI3K/Akt/mTOR overcomes cisplatin resistance in the triple negative breast cancer cell line HCC38
- PMID: 29100507
- PMCID: PMC5670521
- DOI: 10.1186/s12885-017-3695-5
Inhibition of PI3K/Akt/mTOR overcomes cisplatin resistance in the triple negative breast cancer cell line HCC38
Abstract
Background: Widely established targeted therapies directed at triple negative breast cancer (TNBC) are missing. Classical chemotherapy remains the systemic treatment option. Cisplatin has been tested in TNBC but bears the disadvantage of resistance development. The purpose of this study was to identify resistance mechanisms in cisplatin-resistant TNBC cell lines and select targeted therapies based on these findings.
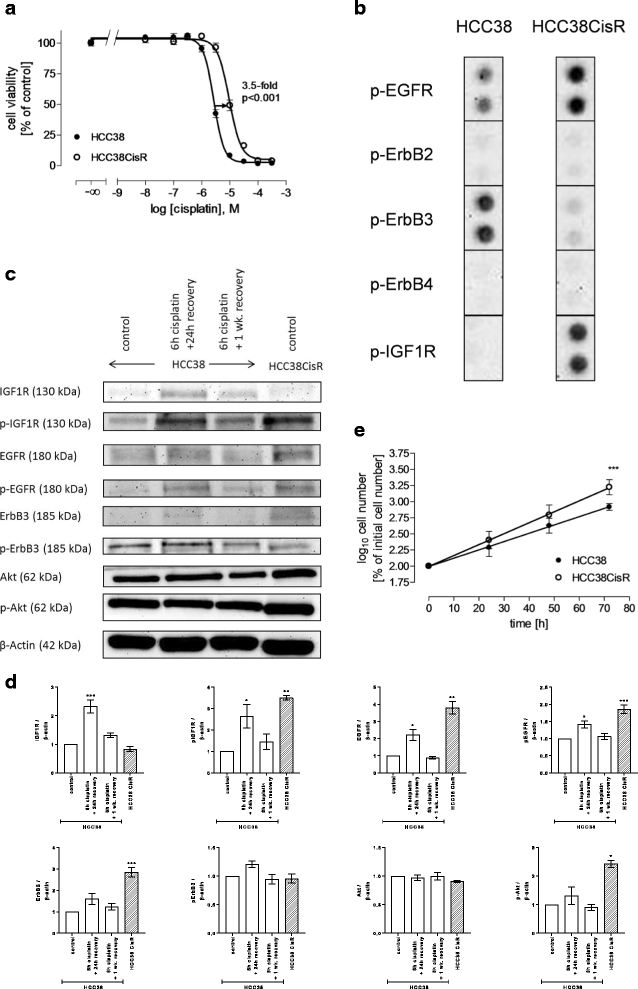
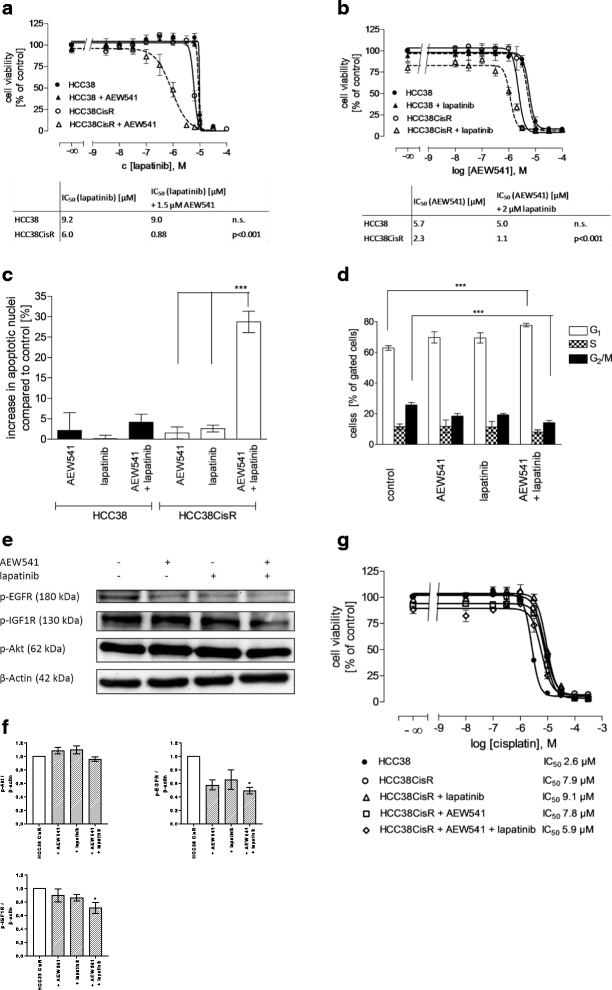
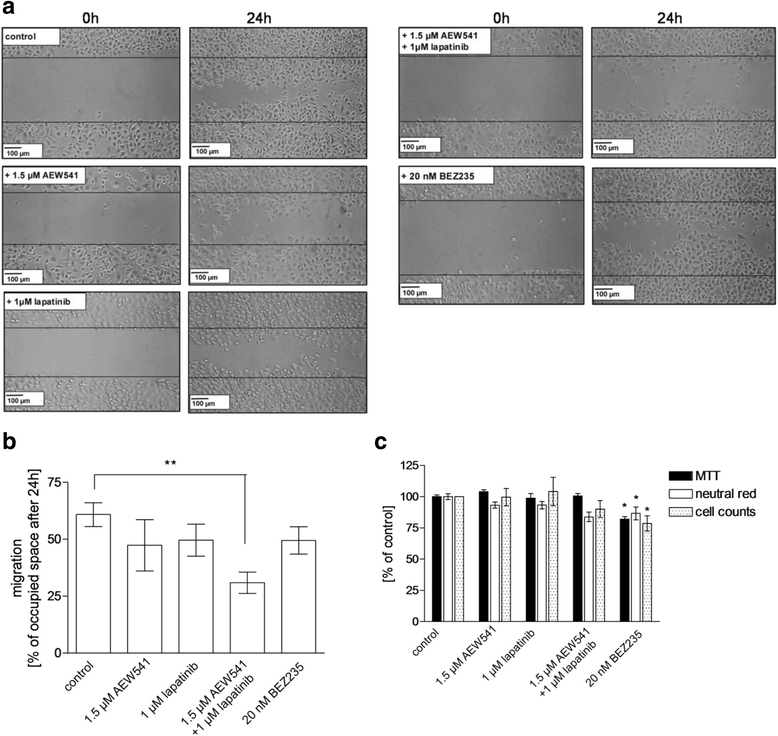
Methods: The TNBC cell lines HCC38 and MDA-MB231 were subjected to intermittent cisplatin treatment resulting in the 3.5-fold cisplatin-resistant subclone HCC38CisR and the 2.1-fold more resistant MDA-MB231CisR. Activation of pro-survival pathways was explored by immunostaining of phospho-receptor tyrosine kinases. Targeted therapies (NVP-AEW541, lapatinib and NVP-BEZ235) against activated pathways were investigated regarding cancer cell growth and cisplatin sensitivity.
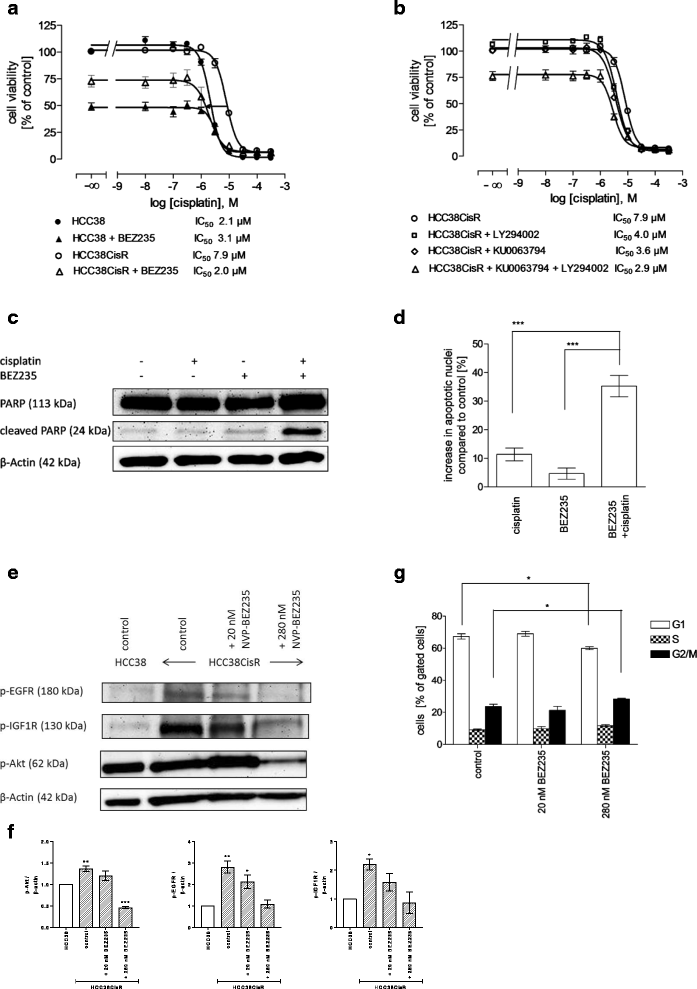
Results: In HCC38CisR and MDA-MB231CisR, phosphorylation of epidermal growth factor receptor (EGFR) and insulin-like growth factor 1 receptor (IGF1R) was observed. In HCC38CisR, treatment with NVP-AEW541 increased potency of lapatinib almost seven-fold, but both compounds could not restore cisplatin sensitivity. However, the dual phosphoinositide 3-kinase (PI3K) and mammalian target of rapamycin (mTOR) inhibitor NVP-BEZ235 acted synergistically with cisplatin in HCC38CisR and fully restored cisplatin sensitivity. Similarly, NVP-BEZ235 increased cisplatin potency in MDA-MB231CisR. Furthermore, NVP-AEW541 in combination with lapatinib restored cisplatin sensitivity in MDA-MB231CisR.
Conclusion: Simultaneous inhibition of EGFR and IGF1R in cisplatin-resistant TNBC cell lines was synergistic regarding inhibition of proliferation and induction of apoptosis. Co-treatment with NVP-BEZ235 or with a combination of NVP-AEW541 and lapatinib restored cisplatin sensitivity and may constitute a targeted treatment option for cisplatin-resistant TNBC.
Keywords: Cisplatin resistance; EGFR; HCC38; IGF1R; Lapatinib; MDA-MB231; NVP-AEW541; NVP-BEZ235; Triple negative breast cancer.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
The PI3K/mTOR dual inhibitor NVP-BEZ235 stimulates mutant p53 degradation to exert anti-tumor effects on triple-negative breast cancer cells.FEBS Open Bio. 2020 Apr;10(4):535-545. doi: 10.1002/2211-5463.12806. Epub 2020 Mar 6. FEBS Open Bio. 2020. PMID: 32027103 Free PMC article.
-
Synergistic antitumor effect of NVP-BEZ235 and CAPE on MDA-MB-231 breast cancer cells.Biomed Pharmacother. 2017 Aug;92:39-45. doi: 10.1016/j.biopha.2017.05.051. Epub 2017 May 18. Biomed Pharmacother. 2017. PMID: 28528184
-
Dual targeting of phosphoinositide 3-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach in human ovarian carcinoma.Clin Cancer Res. 2011 Apr 15;17(8):2373-84. doi: 10.1158/1078-0432.CCR-10-2289. Epub 2011 Mar 3. Clin Cancer Res. 2011. PMID: 21372221 Free PMC article.
-
Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review.Breast Cancer Res Treat. 2018 Jun;169(3):397-406. doi: 10.1007/s10549-018-4697-y. Epub 2018 Feb 7. Breast Cancer Res Treat. 2018. PMID: 29417298 Review.
-
Triple negative breast cancer: shedding light onto the role of pi3k/akt/mtor pathway.Oncotarget. 2016 Sep 13;7(37):60712-60722. doi: 10.18632/oncotarget.10858. Oncotarget. 2016. PMID: 27474173 Free PMC article. Review.
Cited by
-
Mechanisms of Matrix-Induced Chemoresistance of Breast Cancer Cells-Deciphering Novel Potential Targets for a Cell Sensitization.Cancers (Basel). 2018 Dec 6;10(12):495. doi: 10.3390/cancers10120495. Cancers (Basel). 2018. PMID: 30563275 Free PMC article.
-
Silence of α1-Antitrypsin Inhibits Migration and Proliferation of Triple Negative Breast Cancer Cells.Med Sci Monit. 2018 Sep 27;24:6851-6860. doi: 10.12659/MSM.910665. Med Sci Monit. 2018. PMID: 30260937 Free PMC article.
-
Aspirin enhances cisplatin sensitivity of resistant non-small cell lung carcinoma stem-like cells by targeting mTOR-Akt axis to repress migration.Sci Rep. 2019 Nov 15;9(1):16913. doi: 10.1038/s41598-019-53134-0. Sci Rep. 2019. PMID: 31729456 Free PMC article.
-
Identification of a novel S6K1 inhibitor, rosmarinic acid methyl ester, for treating cisplatin-resistant cervical cancer.BMC Cancer. 2019 Aug 6;19(1):773. doi: 10.1186/s12885-019-5997-2. BMC Cancer. 2019. PMID: 31387554 Free PMC article.
-
Synergistic antitumor effect of dual PI3K and mTOR inhibitor NVP-BEZ235 in combination with cisplatin on drug-resistant non-small cell lung cancer cell.Oncol Lett. 2020 Dec;20(6):326. doi: 10.3892/ol.2020.12189. Epub 2020 Oct 5. Oncol Lett. 2020. PMID: 33123242 Free PMC article.
References
-
- von Minckwitz G, Schneeweiss A, Loibl S, Salat C, Denkert C, Rezai M, Blohmer JU, Jackisch C, Paepke S, Gerber B et al: Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014, 15(7):747-756. - PubMed
-
- Isakoff SJ, Mayer EL, He L, Traina TA, Carey LA, Krag KJ, Rugo HS, Liu MC, Stearns V, Come SE, et al. TBCRC009: a multicenter phase II clinical trial of platinum Monotherapy with biomarker assessment in metastatic triple-negative breast cancer. J Clin Oncol. 2015;33(17):1902–1909. doi: 10.1200/JCO.2014.57.6660. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

