Ghrelin therapy improves lung and cardiovascular function in experimental emphysema
- PMID: 29100513
- PMCID: PMC5670513
- DOI: 10.1186/s12931-017-0668-9
Ghrelin therapy improves lung and cardiovascular function in experimental emphysema
Abstract
Background: Emphysema is a progressive disease characterized by irreversible airspace enlargement followed by a decline in lung function. It also causes extrapulmonary effects, such as loss of body mass and cor pulmonale, which are associated with shorter survival and worse clinical outcomes. Ghrelin, a growth-hormone secretagogue, stimulates muscle anabolism, has anti-inflammatory effects, promotes vasodilation, and improves cardiac performance. Therefore, we hypothesized that ghrelin might reduce lung inflammation and remodelling as well as improve lung mechanics and cardiac function in experimental emphysema.
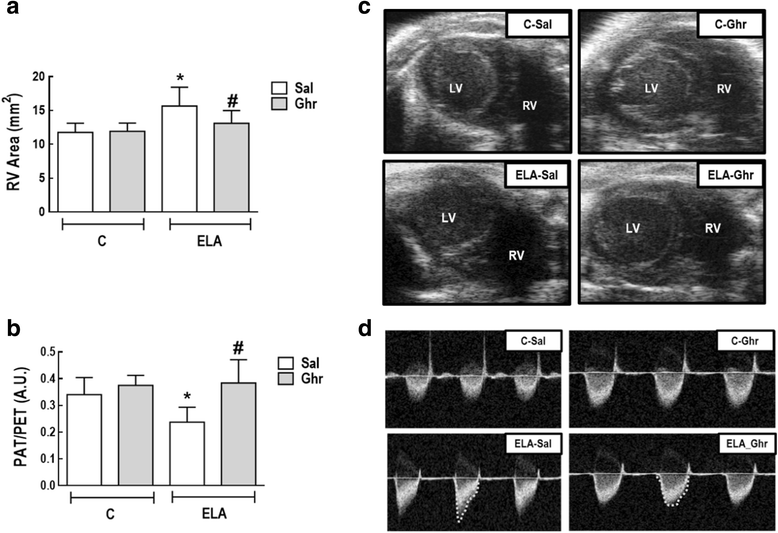
Methods: Forty female C57BL/6 mice were randomly assigned into two main groups: control (C) and emphysema (ELA). In the ELA group (n=20), animals received four intratracheal instillations of pancreatic porcine elastase (PPE) at 1-week intervals. C animals (n=20) received saline alone (50 μL) using the same protocol. Two weeks after the last instillation of saline or PPE, C and ELA animals received ghrelin or saline (n=10/group) intraperitoneally (i.p.) daily, during 3 weeks. Dual-energy X-ray absorptiometry (DEXA), echocardiography, lung mechanics, histology, and molecular biology were analysed.
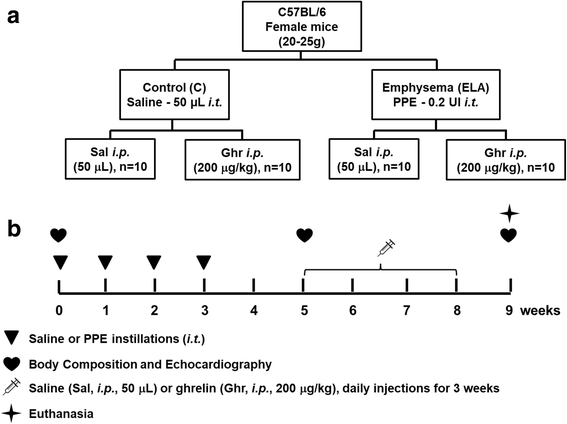
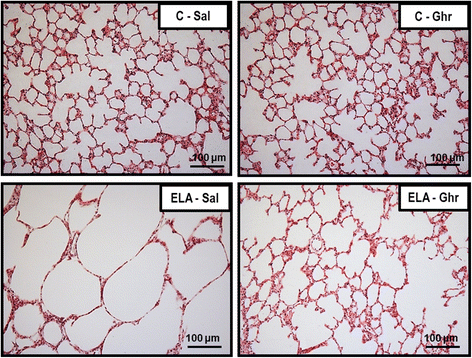
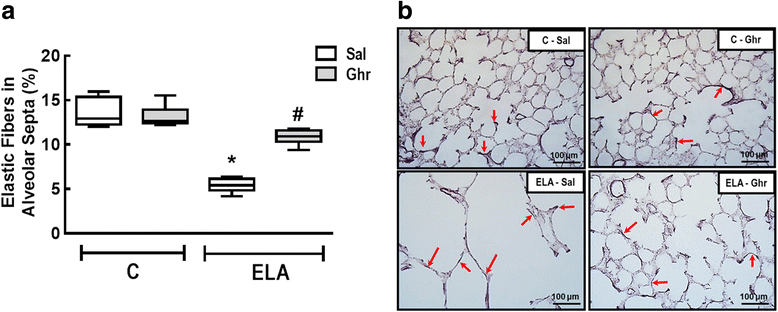
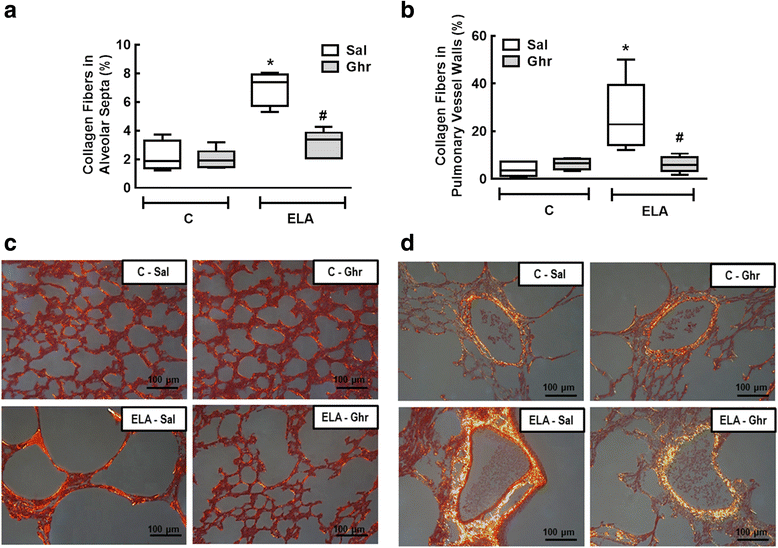
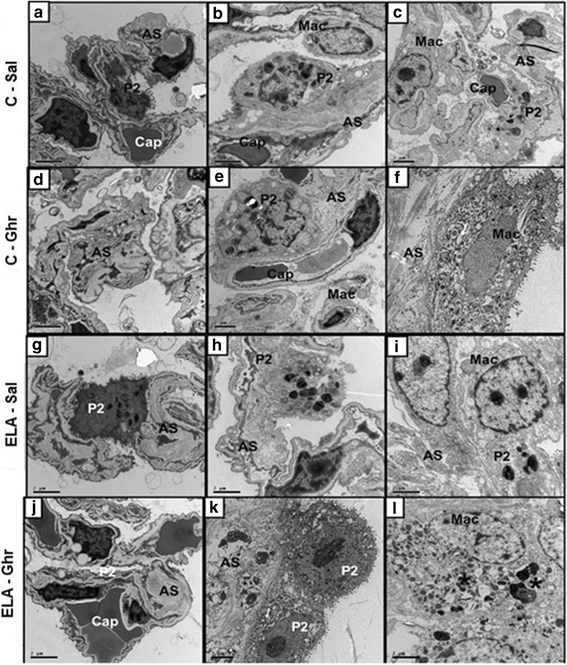
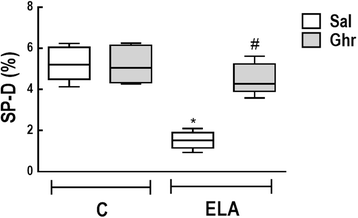
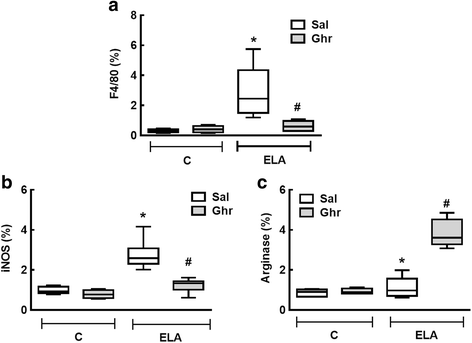
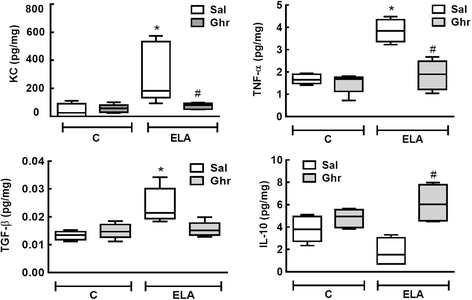
Results: In elastase-induced emphysema, ghrelin treatment decreased alveolar hyperinflation and mean linear intercept, neutrophil infiltration, and collagen fibre content in the alveolar septa and pulmonary vessel wall; increased elastic fibre content; reduced M1-macrophage populations and increased M2 polarization; decreased levels of keratinocyte-derived chemokine (KC, a mouse analogue of interleukin-8), tumour necrosis factor-α, and transforming growth factor-β, but increased interleukin-10 in lung tissue; augmented static lung elastance; reduced arterial pulmonary hypertension and right ventricular hypertrophy on echocardiography; and increased lean mass.
Conclusion: In the elastase-induced emphysema model used herein, ghrelin not only reduced lung damage but also improved cardiac function and increased lean mass. These findings should prompt further studies to evaluate ghrelin as a potential therapy for emphysema.
Keywords: Cardiac function; Experimental emphysema; Ghrelin therapy; Lung function; Lung inflammation; Lung remodelling.
Conflict of interest statement
Ethics approval
This study was approved by the Animal Ethics Committee of the Health Sciences Centre, Federal University of Rio de Janeiro (CEUA: 183/13). All animals received humane care in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the U.S. National Research Council
Consent for publication
Not applicable.
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Characterization of a Mouse Model of Emphysema Induced by Multiple Instillations of Low-Dose Elastase.Front Physiol. 2016 Oct 7;7:457. doi: 10.3389/fphys.2016.00457. eCollection 2016. Front Physiol. 2016. PMID: 27774071 Free PMC article.
-
Impact of one versus two doses of mesenchymal stromal cells on lung and cardiovascular repair in experimental emphysema.Stem Cell Res Ther. 2018 Nov 8;9(1):296. doi: 10.1186/s13287-018-1043-6. Stem Cell Res Ther. 2018. PMID: 30409216 Free PMC article.
-
Endotoxin-Induced Emphysema Exacerbation: A Novel Model of Chronic Obstructive Pulmonary Disease Exacerbations Causing Cardiopulmonary Impairment and Diaphragm Dysfunction.Front Physiol. 2019 May 28;10:664. doi: 10.3389/fphys.2019.00664. eCollection 2019. Front Physiol. 2019. PMID: 31191356 Free PMC article.
-
Can exogenously administered hyaluronan improve respiratory function in patients with pulmonary emphysema?Chest. 2004 Jan;125(1):288-92. doi: 10.1378/chest.125.1.288. Chest. 2004. PMID: 14718453 Review.
-
Contributions of retinoids to the generation and repair of the pulmonary alveolus.Chest. 2002 May;121(5 Suppl):206S-208S. doi: 10.1378/chest.121.5_suppl.206s. Chest. 2002. PMID: 12010853 Review.
Cited by
-
Exploring the Antifibrotic Mechanisms of Ghrelin: Modulating TGF-β Signalling in Organ Fibrosis.Expert Rev Mol Med. 2024 Nov 21;27:e8. doi: 10.1017/erm.2024.38. Expert Rev Mol Med. 2024. PMID: 39569809 Free PMC article. Review.
-
Potential Applications for Growth Hormone Secretagogues Treatment of Amyotrophic Lateral Sclerosis.Curr Neuropharmacol. 2023;21(12):2376-2394. doi: 10.2174/1570159X20666220915103613. Curr Neuropharmacol. 2023. PMID: 36111771 Free PMC article.
-
Inhibition of DLL4/Notch Signaling Pathway Promotes M2 Polarization and Cell Proliferation in Pulmonary Arterial Hypertension.ACS Omega. 2024 Aug 26;9(36):37923-37933. doi: 10.1021/acsomega.4c04307. eCollection 2024 Sep 10. ACS Omega. 2024. PMID: 39281910 Free PMC article.
-
Ghrelin attenuates inflammation in diabetic lung disease by TLR4 pathway in vivo and in vitro.BMJ Open Diabetes Res Care. 2023 Apr;11(2):e003027. doi: 10.1136/bmjdrc-2022-003027. BMJ Open Diabetes Res Care. 2023. PMID: 37085277 Free PMC article.
-
A Lactobacillus Combination Ameliorates Lung Inflammation in an Elastase/LPS-induced Mouse Model of Chronic Obstructive Pulmonary Disease.Probiotics Antimicrob Proteins. 2024 Jun 12. doi: 10.1007/s12602-024-10300-9. Online ahead of print. Probiotics Antimicrob Proteins. 2024. PMID: 38865030
References
-
- Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur Respir J. 2017:49. - PubMed
-
- Janoff A. Elastases and emphysema. Current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis. 1985;132:417–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

