Effect of ketamine combined with magnesium sulfate in neuropathic pain patients (KETAPAIN): study protocol for a randomized controlled trial
- PMID: 29100524
- PMCID: PMC5670712
- DOI: 10.1186/s13063-017-2254-3
Effect of ketamine combined with magnesium sulfate in neuropathic pain patients (KETAPAIN): study protocol for a randomized controlled trial
Abstract
Background: Neuropathic pain is difficult to treat, and the efficacy of recommended drugs remains limited. N-methyl-D-aspartate receptors are implicated, and antagonists are a pharmacological option. Ketamine is widely used in French pain clinics, but without consensus or recommendations. Furthermore, the association of ketamine with magnesium has been poorly studied. The aim of the present study is to evaluate the benefit of ketamine with or without magnesium in refractory neuropathic pain.
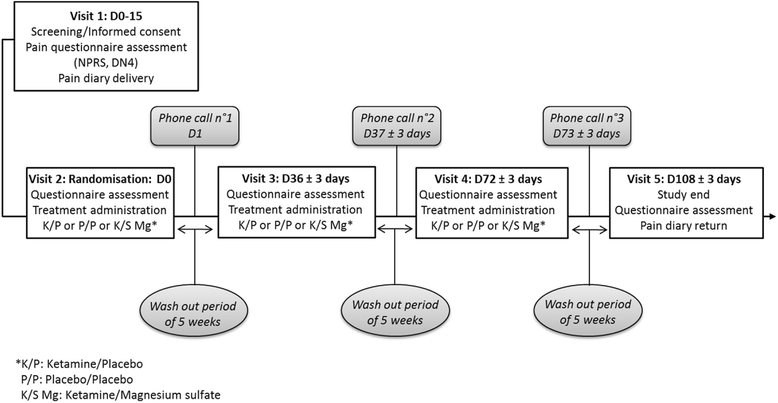
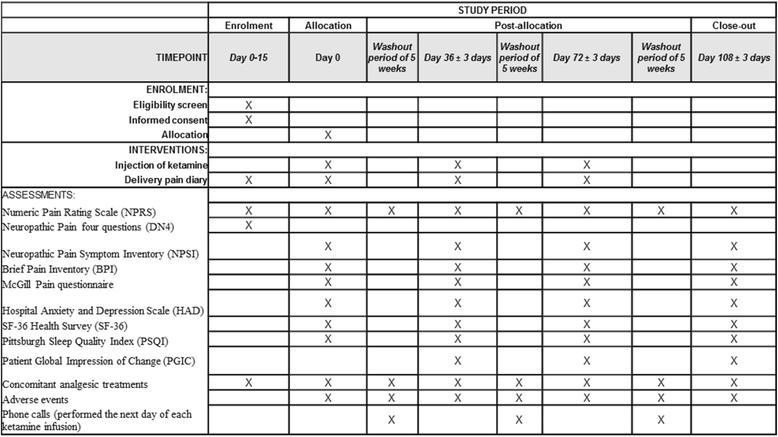
Methods/design: A randomized, double-blind, crossover, placebo-controlled study will be performed in Clermont-Ferrand University Hospital, Clermont-Ferrand, France. The aim is to evaluate the effect of ketamine with or without magnesium in 22 patients with neuropathic pain. Intravenous ketamine/placebo, ketamine/magnesium sulfate, or placebo/placebo will be administered consecutively to each patient, in random order, once at 5-week intervals. The primary endpoint is the AUC of pain intensity assessed on a 0-10 Numeric Pain Rating Scale for a 5-week period. Data analysis will be performed on an intention-to-treat basis, and all statistical tests (except primary analysis) will be performed with an α risk of 5% (two-sided).
Discussion: Considering the poor efficacy of the drugs available for neuropathic pain, ketamine with or without magnesium sulfate may be a valuable therapeutic option that needs to be standardized.
Trial registration: EudraCT number- 2015-000142-29 . Registered on April 9, 2015; version 1.4.
Keywords: Ketamine; Magnesium sulfate; N-methyl-D-aspartate receptor; Neuropathic pain; Placebo.
Conflict of interest statement
Ethics approval and consent to participate
The study received approval from the French Research Ethics Committee on April 13, 2015 (IRB number AU 1173). The trial is registered with ClinicalTrials.gov (NCT0246751). Patients meeting the inclusion criteria will sign a consent form after receiving oral and written information.
Consent for publication
All authors have consented to publication of this article.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Finnerup NB, Haroutounian S, Kamerman P, Baron R, Bennett DL, Bouhassira D, Cruccu G, Freeman R, Hansson P, Nurmikko T, Raja SN, Rice AS, Serra J, Smith BH, Treede RD, Jensen TS. Neuropathic pain: an updated grading system for research and clinical practice. Pain. 2016;157:1599–606. doi: 10.1097/j.pain.0000000000000492. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous