Efficacy of trivalent influenza vaccine against laboratory-confirmed influenza among young children in a randomized trial in Bangladesh
- PMID: 29100706
- PMCID: PMC5723570
- DOI: 10.1016/j.vaccine.2017.10.074
Efficacy of trivalent influenza vaccine against laboratory-confirmed influenza among young children in a randomized trial in Bangladesh
Abstract
Background: Few trials have evaluated influenza vaccine efficacy (VE) in young children, a group particularly vulnerable to influenza complications. We aimed to estimate VE against influenza in children aged <2 years in Bangladesh; a subtropical setting, where influenza circulation can be irregular.
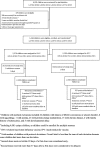
Methods: Children aged 6-23 months were enrolled 1:1 in a parallel, double-blind, randomized controlled trial of trivalent inactivated influenza vaccine (IIV3) versus inactivated polio vaccine (IPV); conducted August 2010-March 2014 in Dhaka, Bangladesh. Children received two pediatric doses of vaccine, one month apart, and were followed for one year for febrile and respiratory illness. Field assistants conducted weekly home-based, active surveillance and ill children were referred to the study clinic for clinical evaluation and nasopharyngeal wash specimen collection. Analysis included all children who received a first vaccine dose and compared yearly incidence of reverse transcription polymerase chain reaction (RT-PCR)-confirmed influenza between trial arms. The VE was estimated as 1-(rate ratio of illness) × 100%, using unadjusted Poisson regression. The trial was registered with ClinicalTrials.gov, number NCT01319955.
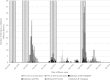
Results: Across four vaccination rounds, 4081 children were enrolled and randomized, contributing 2576 child-years of observation to the IIV3 arm and 2593 child-years to the IPV arm. Influenza incidence was 10 episodes/100 child-years in the IIV3 arm and 15 episodes/100 child-years in the IPV arm. Overall, the VE was 31% (95% confidence interval 18, 42%) against any RT-PCR-confirmed influenza. The VE varied by season, but was similar by influenza type/subtype and participant age and sex.
Conclusions: Vaccination of young children with IIV3 provided a significant reduction in laboratory-confirmed influenza; however, exploration of additional influenza vaccine strategies, such as adjuvanted vaccines or standard adult vaccine doses, is warranted to find more effective influenza vaccines for young children in low-income countries.
Keywords: Children; Clinical trial; Influenza; Vaccine.
Published by Elsevier Ltd.
Figures


References
-
- Nair H., Brooks W.A., Katz M., Roca A., Berkley J.A., Madhi S.A. Global burden of respiratory infections due to seasonal influenza in young children: a systematic review and meta-analysis. Lancet. 2011;378:1917–1930. - PubMed
-
- World Health Organization Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012;87:461–476. - PubMed
-
- Hoberman A., Greenberg D.P., Paradise J.L., Rockette H.E., Lave J.R., Kearney D.H. Effectiveness of inactivated influenza vaccine in preventing acute otitis media in young children: a randomized controlled trial. JAMA. 2003;290:1608–1616. - PubMed
-
- Vesikari T., Knuf M., Wutzler P., Karvonen A., Kieninger-Baum D., Schmitt H.J. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365:1406–1416. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

