Conditional loss of Spata7 in photoreceptors causes progressive retinal degeneration in mice
- PMID: 29100828
- PMCID: PMC5756513
- DOI: 10.1016/j.exer.2017.10.015
Conditional loss of Spata7 in photoreceptors causes progressive retinal degeneration in mice
Erratum in
-
Corrigendum to "Conditional loss of Spata7 in photoreceptors causes progressive retinal degeneration in mice" [Exp. Eye Res. 166 (2018) 120-130].Exp Eye Res. 2018 Jun;171:119. doi: 10.1016/j.exer.2018.03.011. Epub 2018 Mar 24. Exp Eye Res. 2018. PMID: 29579643 No abstract available.
Abstract
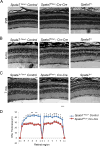
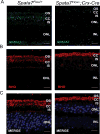
The mammalian retina consists of multiple cell layers including photoreceptor cells, which are light sensing neurons that play essential functions in the visual process. Previously, we identified mutations in SPATA7, encoding spermatogenesis associated protein 7, in families with Leber Congenital Amaurosis (LCA) and juvenile Retinitis Pigmentosa (RP), and showed that Spata7 null mice recapitulate the human disease phenotype of retinal degeneration. SPATA7 is expressed in the connecting cilium of photoreceptor (PR) cells in the mouse retina, as well as in retinal pigment epithelium (RPE) cells, but the functional role of Spata7 in the RPE remains unknown. To investigate whether Spata7 is required in PRs, the RPE, or both, we conditionally knocked out Spata7 in photoreceptors and RPE cells using Crx-Cre and Best1-Cre transgenic mouse lines, respectively. In Spata7 photoreceptor-specific conditional (cKO) mice, both rod and cone photoreceptor dysfunction and degeneration is observed, characterized by progressive thinning of the outer nuclear layer and reduced response to light; however, RPE-specific deletion of Spata7 does not impair retinal function or cell survival. Furthermore, our findings show that both Rhodopsin and RPGRIP1 are mislocalized in the Spata7Flox/-; Crx-Cre cKO mice, suggesting that loss of Spata7 in photoreceptors alone can result in altered trafficking of these proteins in the connecting cilium. Together, our findings suggest that loss of Spata7 in photoreceptors alone is sufficient to cause photoreceptor degeneration, but its function in the RPE is not required for photoreceptor survival; therefore, loss of Spata7 in photoreceptors alters both rod and cone function and survival, consistent with the clinical phenotypes observed in LCA and RP patients with mutations in SPATA7.
Keywords: Conditional knockout; Leber Congenital Amaurosis; Photoreceptors; RPE; Retinal degeneration; Retinal function; Retinitis Pigmentosa; SPATA7.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures





Similar articles
-
Spata7 is a retinal ciliopathy gene critical for correct RPGRIP1 localization and protein trafficking in the retina.Hum Mol Genet. 2015 Mar 15;24(6):1584-601. doi: 10.1093/hmg/ddu573. Epub 2014 Nov 14. Hum Mol Genet. 2015. PMID: 25398945 Free PMC article.
-
Conditional loss of Kcnj13 in the retinal pigment epithelium causes photoreceptor degeneration.Exp Eye Res. 2018 Nov;176:219-226. doi: 10.1016/j.exer.2018.07.014. Epub 2018 Jul 25. Exp Eye Res. 2018. PMID: 30009826 Free PMC article.
-
AAV8(Y733F)-mediated gene therapy in a Spata7 knockout mouse model of Leber congenital amaurosis and retinitis pigmentosa.Gene Ther. 2015 Aug;22(8):619-27. doi: 10.1038/gt.2015.42. Epub 2015 May 12. Gene Ther. 2015. PMID: 25965394 Free PMC article.
-
Rhodopsin-mediated retinitis pigmentosa.Prog Mol Biol Transl Sci. 2009;88:1-31. doi: 10.1016/S1877-1173(09)88001-0. Epub 2009 Oct 7. Prog Mol Biol Transl Sci. 2009. PMID: 20374723 Review.
-
PRCD Is a Small Disc-Specific Rhodopsin-Binding Protein of Unknown Function.Adv Exp Med Biol. 2019;1185:531-535. doi: 10.1007/978-3-030-27378-1_87. Adv Exp Med Biol. 2019. PMID: 31884666 Review.
Cited by
-
Proteasome-Mediated Regulation of Cdhr1a by Siah1 Modulates Photoreceptor Development and Survival in Zebrafish.Front Cell Dev Biol. 2020 Nov 23;8:594290. doi: 10.3389/fcell.2020.594290. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33330480 Free PMC article.
-
Gene Therapy Rescues Retinal Degeneration in Receptor Expression-Enhancing Protein 6 Mutant Mice.Hum Gene Ther. 2019 Mar;30(3):302-315. doi: 10.1089/hum.2018.078. Epub 2018 Oct 16. Hum Gene Ther. 2019. PMID: 30101608 Free PMC article.
-
Impairment of photoreceptor ribbon synapses in a novel Pomt1 conditional knockout mouse model of dystroglycanopathy.Sci Rep. 2018 Jun 4;8(1):8543. doi: 10.1038/s41598-018-26855-x. Sci Rep. 2018. PMID: 29867208 Free PMC article.
-
Mouse Models of Inherited Retinal Degeneration with Photoreceptor Cell Loss.Cells. 2020 Apr 10;9(4):931. doi: 10.3390/cells9040931. Cells. 2020. PMID: 32290105 Free PMC article. Review.
-
Impaired ABCA1/ABCG1-mediated lipid efflux in the mouse retinal pigment epithelium (RPE) leads to retinal degeneration.Elife. 2019 Mar 13;8:e45100. doi: 10.7554/eLife.45100. Elife. 2019. PMID: 30864945 Free PMC article.
References
-
- Agrawal SA, Burgoyne T, Eblimit A, Bellingham J, Parfitt DA, Lane A, Nichols R, Asomugha C, Hayes MJ, Munro PM, Xu M, Wang K, Futter CE, Li Y, Chen R, Cheetham ME. REEP6 Deficiency Leads to Retinal Degeneration through Disruption of ER Homeostasis and Protein Trafficking. Hum. Mol. Genet 2017 - PMC - PubMed
-
- Bok D. Contributions of genetics to our understanding of inherited monogenic retinal diseases and age-related macular degeneration. Arch. Ophthalmol. 2007;125:160–164. - PubMed
-
- Boylan JP, Wright AF. Identification of a novel protein interacting with RPGR. Hum. Mol. Genet. 2000;9:2085–2093. - PubMed
-
- Eblimit A, Nguyen TM, Chen Y, Esteve-Rudd J, Zhong H, Letteboer S, Van Reeuwijk J, Simons DL, Ding Q, Wu KM, Li Y, Van Beersum S, Moayedi Y, Xu H, Pickard P, Wang K, Gan L, Wu SM, Williams DS, Mardon G, Roepman R, Chen R. Spata7 is a retinal ciliopathy gene critical for correct RPGRIP1 localization and protein trafficking in the retina. Hum. Mol. Genet. 2015;24:1584–1601. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

