Hyperoxia-mediated transcriptional activation of cytochrome P4501A1 (CYP1A1) and decreased susceptibility to oxygen-mediated lung injury in newborn mice
- PMID: 29101037
- PMCID: PMC5743196
- DOI: 10.1016/j.bbrc.2017.10.166
Hyperoxia-mediated transcriptional activation of cytochrome P4501A1 (CYP1A1) and decreased susceptibility to oxygen-mediated lung injury in newborn mice
Abstract
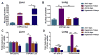
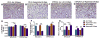
Hyperoxia contributes to the development of bronchopulmonary dysplasia (BPD) in premature infants. In this study, we tested the hypothesis that newborn transgenic mice carrying the human CYP1A1-Luc promoter will display transcriptional activation of the human CYP1A1 promoter in vivo upon exposure to hyperoxia, and that these mice will be less susceptible to hyperoxic lung injury and alveolar simplification than similarly exposed wild type (WT) mice. Newborn WT (CD-1) or transgenic mice carrying a 13.2 kb human CYP1A1 promoter and the luciferase (Luc) reporter gene (CYP1A1-luc) were maintained in room air or exposed to hyperoxia (85% O2) for 7-14 days. Hyperoxia exposure of CYP1A1-Luc mice for 7 and 14 days resulted in 4- and 30-fold increases, respectively, in hepatic Luc (CYP1A1) expression, compared to room air controls. In lung, hyperoxia caused a 2-fold induction of reporter Luc at 7 days, but the induction declined after 14 days. The newborn CYP1A1-Luc mice were less susceptible to lung injury and alveolar simplification than similarly exposed wild type (WT) CD-1 mice. Also, the CYP1A1-Luc mice showed increased levels of hepatic and pulmonary CYP1A1 expression and hepatic CYP1A2 activity after hyperoxia exposure. Hyperoxia also increased NADP(H) quinone reductase (NQO1) pulmonary gene expression in both CD-1 and CYP1A1-Luc mice at both time points, but this was more pronounced in the latter at 14 days. Our results support the hypothesis that hyperoxia activates the human CYP1A1 promoter in newborn mice, and that increased endogenous expression of CYP1A1 and NADP(H) quinone reductase (NQO1) contributes to the decreased susceptibilities to hyperoxic lung injury in the transgenic animals. This is the first report providing evidence of hyperoxia-mediated transcriptional activation of the human CYP1A1 promoter in newborn mice, and this in conjunction with decreased lung injury, suggests that these phenomena have important implications for BPD.
Keywords: Bronchopulmonary dysplasia (BPD); Cytochrome P450; Human CYP1A1-luciferase; Hyperoxia; Lung injury; Newborn.
Copyright © 2017. Published by Elsevier Inc.
Figures
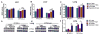
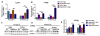
Similar articles
-
Augmented oxygen-mediated transcriptional activation of cytochrome P450 (CYP)1A expression and increased susceptibilities to hyperoxic lung injury in transgenic mice carrying the human CYP1A1 or mouse 1A2 promoter in vivo.Biochem Biophys Res Commun. 2011 Apr 1;407(1):79-85. doi: 10.1016/j.bbrc.2011.02.113. Epub 2011 Mar 6. Biochem Biophys Res Commun. 2011. PMID: 21362406 Free PMC article.
-
Newborn Mice Lacking the Gene for Cyp1a1 Are More Susceptible to Oxygen-Mediated Lung Injury, and Are Rescued by Postnatal β-Naphthoflavone Administration: Implications for Bronchopulmonary Dysplasia in Premature Infants.Toxicol Sci. 2017 May 1;157(1):260-271. doi: 10.1093/toxsci/kfx036. Toxicol Sci. 2017. PMID: 28201809 Free PMC article.
-
Quercetin attenuates the hyperoxic lung injury in neonatal mice: Implications for Bronchopulmonary dysplasia (BPD).Food Chem Toxicol. 2018 Apr;114:23-33. doi: 10.1016/j.fct.2018.02.026. Epub 2018 Feb 9. Food Chem Toxicol. 2018. PMID: 29432836
-
Hyperoxia-induced bronchopulmonary dysplasia: better models for better therapies.Dis Model Mech. 2021 Feb 23;14(2):dmm047753. doi: 10.1242/dmm.047753. Dis Model Mech. 2021. PMID: 33729989 Free PMC article. Review.
-
The Central Role of Cytochrome P450 Reductase (CPR) in Hyperoxic Lung Injury.Expert Opin Drug Metab Toxicol. 2025 May;21(5):589-598. doi: 10.1080/17425255.2025.2470808. Epub 2025 Feb 25. Expert Opin Drug Metab Toxicol. 2025. PMID: 39992710 Review.
Cited by
-
Oxygen Toxicity to the Immature Lung-Part I: Pathomechanistic Understanding and Preclinical Perspectives.Int J Mol Sci. 2021 Oct 12;22(20):11006. doi: 10.3390/ijms222011006. Int J Mol Sci. 2021. PMID: 34681665 Free PMC article. Review.
-
Genetic and Epidemiological Similarities, and Differences Between Postoperative Intraperitoneal Adhesion Development and Other Benign Fibro-proliferative Disorders.Reprod Sci. 2022 Nov;29(11):3055-3077. doi: 10.1007/s43032-021-00726-9. Epub 2021 Sep 13. Reprod Sci. 2022. PMID: 34515982 Review.
-
Is There a Genetic Predisposition to Postoperative Adhesion Development?Reprod Sci. 2021 Aug;28(8):2076-2086. doi: 10.1007/s43032-020-00356-7. Epub 2020 Oct 22. Reprod Sci. 2021. PMID: 33090376 Free PMC article. Review.
References
-
- Northway WH, Jr, Rosan RC. Radiographic features of pulmonary oxygen toxicity in the newborn: bronchopulmonary dysplasia. Radiology. 1968;91:49–58. - PubMed
-
- Owen LS, Manley BJ, Davis PG, Doyle LW. The evolution of modern respiratory care for preterm infants. Lancet. 2017 Apr 22;389(10079):1649–1659. - PubMed
-
- Bland RD, Coalson JJ. Chronic Lung Disease in Early Infancy. M. Dekker; New York: 2000.
-
- Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. - PubMed
-
- Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, Bauer CR, Donovan EF, Korones SB, Laptook AR, Lemons JA, Oh W, Papile LA, Shankaran S, Stevenson DK, Tyson JE, Poole WK. Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol. 2007;196:147e141–148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

