Smartphone Cognitive Behavioral Therapy as an Adjunct to Pharmacotherapy for Refractory Depression: Randomized Controlled Trial
- PMID: 29101095
- PMCID: PMC5695656
- DOI: 10.2196/jmir.8602
Smartphone Cognitive Behavioral Therapy as an Adjunct to Pharmacotherapy for Refractory Depression: Randomized Controlled Trial
Erratum in
-
Correction: Smartphone Cognitive Behavioral Therapy as an Adjunct to Pharmacotherapy for Refractory Depression: Randomized Controlled Trial.J Med Internet Res. 2018 Aug 31;20(8):e11702. doi: 10.2196/11702. J Med Internet Res. 2018. PMID: 30168797 Free PMC article.
Abstract
Background: In the treatment of major depression, antidepressants are effective but not curative. Cognitive behavioral therapy (CBT) is also effective, alone or in combination with pharmacotherapy, but accessibility is a problem.
Objective: The aim is to evaluate the effectiveness of a smartphone CBT app as adjunctive therapy among patients with antidepressant-resistant major depression.
Methods: A multisite, assessor-masked, parallel-group randomized controlled trial was conducted in 20 psychiatric clinics and hospitals in Japan. Participants were eligible if they had a primary diagnosis of major depression and were antidepressant-refractory after taking one or more antidepressants at an adequate dosage for four or more weeks. After a 1-week run-in in which participants started the medication switch and had access to the welcome session of the app, patients were randomized to medication switch alone or to medication switch plus smartphone CBT app via the centralized Web system. The smartphone app, called Kokoro-app ("kokoro" means "mind" in Japanese), included sessions on self-monitoring, behavioral activation, and cognitive restructuring presented by cartoon characters. The primary outcome was depression severity as assessed by masked telephone assessors with the Patient Health Questionnaire-9 (PHQ-9) at week 9. The secondary outcomes included the Beck Depression Inventory-II (BDI-II) and Frequency, Intensity, and Burden of Side Effects Ratings (FIBSER).
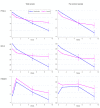
Results: In the total sample (N=164), 81 participants were allocated to the smartphone CBT in addition to medication change and 83 to medication change alone. In the former group, all but one participant (80/81, 99%) completed at least half, and 71 (88%) completed at least six of eight sessions. In the intention-to-treat analysis, patients allocated the CBT app scored 2.48 points (95% CI 1.23-3.72, P<.001; standardized mean difference 0.40) lower on PHQ-9 than the control at week 9. The former group also scored 4.1 points (95% CI 1.5-6.6, P=.002) lower on BDI-II and 0.76 points (95% CI -0.05 to 1.58, P=.07) lower on FIBSER. In the per-protocol sample (comfortable with the smartphone app, still symptomatic, and adherent to medication with mild or less side effects after run-in), the intervention group (n=60) scored 1.72 points (95% CI 0.25-3.18, P=.02) lower on PHQ-9, 3.2 points (95% CI -0.01 to 6.3, P=.05) lower on BDI-II, and 0.75 points (95% CI 0.03-1.47, P=.04) lower on FIBSER than the control (n=57). The treatment benefits were maintained up to week 17.
Conclusions: This is the first study to demonstrate the effectiveness of a smartphone CBT in the treatment of clinically diagnosed depression. Given the merits of the mobile mental health intervention, including accessibility, affordability, quality control, and effectiveness, it is clinically worthwhile to consider adjunctive use of a smartphone CBT app when treating patients with antidepressant-resistant depression. Research into its effectiveness in wider clinical contexts is warranted.
Trial registration: Japanese Clinical Trials Registry UMIN CTR 000013693; https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ ctr_view.cgi?recptno=R000015984 (Archived by WebCite at http://www.webcitation.org/6u6pxVwik).
Keywords: cognitive behavioral therapy; eHealth; major depressive disorder; mobile phone app; pharmacotherapy-resistant depression.
©Akio Mantani, Tadashi Kato, Toshi A Furukawa, Masaru Horikoshi, Hissei Imai, Takahiro Hiroe, Bun Chino, Tadashi Funayama, Naohiro Yonemoto, Qi Zhou, Nao Kawanishi. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 03.11.2017.
Conflict of interest statement
Conflicts of Interest: AM has received lecture fees from Mochida, Eli Lilly, and Meiji. TK has received lecture fees from Eli Lilly and Mitsubishi-Tanabe, and has contracted research with GlaxoSmithKline, MSD, and Mitsubishi-Tanabe. TAF has received lecture fees from Eli Lilly, Janssen, Meiji, MSD, Otsuka, Pfizer, and Mitsubishi-Tanabe, and consultancy fees from Takeda Science Foundation. He has received research support from Mochida and Mitsubishi-Tanabe. TH has received lecture fees from Otsuka and MSD. BC has received lecture fees from Eli Lilly, Meiji, and Mitsubishi-Tanabe. TF has received lecture fees from Eli Lilly, Meiji, MSD, Otsuka, Pfizer, and Mitsubishi-Tanabe. TAF and MH developed the Kokoro-app. All the other authors declare that they have no competing interests.
Figures

Similar articles
-
Cognitive and Behavioral Skills Exercises Completed by Patients with Major Depression During Smartphone Cognitive Behavioral Therapy: Secondary Analysis of a Randomized Controlled Trial.JMIR Ment Health. 2018 Jan 11;5(1):e4. doi: 10.2196/mental.9092. JMIR Ment Health. 2018. PMID: 29326098 Free PMC article.
-
Adding smartphone-based cognitive-behavior therapy to pharmacotherapy for major depression (FLATT project): study protocol for a randomized controlled trial.Trials. 2015 Jul 7;16:293. doi: 10.1186/s13063-015-0805-z. Trials. 2015. PMID: 26149441 Free PMC article. Clinical Trial.
-
Web-Based Cognitive Behavioral Therapy Blended With Face-to-Face Sessions for Major Depression: Randomized Controlled Trial.J Med Internet Res. 2018 Sep 21;20(9):e10743. doi: 10.2196/10743. J Med Internet Res. 2018. PMID: 30249583 Free PMC article. Clinical Trial.
-
Novel Augmentation Strategies in Major Depression.Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review.
-
Behavioral Activation Contributed to the Total Reduction of Depression Symptoms in the Smartphone-based Cognitive Behavioral Therapy: A Secondary Analysis of a Randomized, Controlled Trial.Innov Clin Neurosci. 2020 Jul 1;17(7-9):21-25. Innov Clin Neurosci. 2020. PMID: 33520400 Free PMC article. Review.
Cited by
-
App-Based Interventions for Moderate to Severe Depression: A Systematic Review and Meta-Analysis.JAMA Netw Open. 2023 Nov 1;6(11):e2344120. doi: 10.1001/jamanetworkopen.2023.44120. JAMA Netw Open. 2023. PMID: 37983028 Free PMC article.
-
Effectiveness of a Mobile Application for Postpartum Depression Self-Management: Evidence from a Randomised Controlled Trial in South Korea.Healthcare (Basel). 2022 Oct 31;10(11):2185. doi: 10.3390/healthcare10112185. Healthcare (Basel). 2022. PMID: 36360528 Free PMC article.
-
Review of Use of Asynchronous Technologies Incorporated in Mental Health Care.Curr Psychiatry Rep. 2018 Aug 28;20(10):85. doi: 10.1007/s11920-018-0954-3. Curr Psychiatry Rep. 2018. PMID: 30155593 Review.
-
Optimization of smartphone psychotherapy for depression and anxiety among patients with cancer using the multiphase optimization strategy (MOST) framework and decentralized clinical trial system (SMartphone Intervention to LEssen depression/Anxiety and GAIN resilience: SMILE AGAIN project): a protocol for a randomized controlled trial.Trials. 2023 May 22;24(1):344. doi: 10.1186/s13063-023-07307-y. Trials. 2023. PMID: 37217965 Free PMC article.
-
Optimizing smartphone psychotherapy for depressive symptoms in patients with cancer: Multiphase optimization strategy using a decentralized multicenter randomized clinical trial (J-SUPPORT 2001 Study).Psychiatry Clin Neurosci. 2024 Jun;78(6):353-361. doi: 10.1111/pcn.13657. Epub 2024 Mar 11. Psychiatry Clin Neurosci. 2024. PMID: 38468404 Free PMC article. Clinical Trial.
References
-
- Bromet E, Andrade LH, Hwang I, Sampson NA, Alonso J, de Girolamo G, de Graaf R, Demyttenaere K, Hu C, Iwata N, Karam AN, Kaur J, Kostyuchenko S, Lépine J, Levinson D, Matschinger H, Mora MEM, Browne MO, Posada-Villa J, Viana MC, Williams DR, Kessler RC. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011 Jul 26;9:90. doi: 10.1186/1741-7015-9-90. https://bmcmedicine.biomedcentral.com/articles/10.1186/1741-7015-9-90 - DOI - DOI - PMC - PubMed
-
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013 Nov;10(11):e1001547. doi: 10.1371/journal.pmed.1001547. http://dx.plos.org/10.1371/journal.pmed.1001547 - DOI - DOI - PMC - PubMed
-
- Bloom D, Cafiero E, Jane-Llopis E, Abrahams-Gessel S, Bloom L, Fathima S. The Global Economic Burden of Noncommunicable Diseases. Geneva: World Economic Forum; 2011.
-
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006 Nov;3(11):e442. doi: 10.1371/journal.pmed.0030442. http://dx.plos.org/10.1371/journal.pmed.0030442 - DOI - DOI - PMC - PubMed
-
- Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, Angermeyer MC, Bernert S, de Girolamo G, Morosini P, Polidori G, Kikkawa T, Kawakami N, Ono Y, Takeshima T, Uda H, Karam EG, Fayyad JA, Karam AN, Mneimneh ZN, Medina-Mora ME, Borges G, Lara C, de Graaf R, Ormel J, Gureje O, Shen Y, Huang Y, Zhang M, Alonso J, Haro JM, Vilagut G, Bromet EJ, Gluzman S, Webb C, Kessler RC, Merikangas KR, Anthony JC, Von Korff MR, Wang PS, Brugha TS, Aguilar-Gaxiola S, Lee S, Heeringa S, Pennell B, Zaslavsky AM, Ustun TB, Chatterji S. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004 Jun 2;291(21):2581–2590. doi: 10.1001/jama.291.21.2581. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

