Cytokine-Mediated Regulation of CD8 T-Cell Responses During Acute and Chronic Viral Infection
- PMID: 29101105
- PMCID: PMC6314063
- DOI: 10.1101/cshperspect.a028464
Cytokine-Mediated Regulation of CD8 T-Cell Responses During Acute and Chronic Viral Infection
Abstract
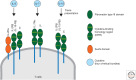
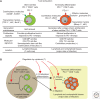
The common γ-chain cytokines, interleukin (IL)-2, IL-7, and IL-15, regulate critical aspects of antiviral CD8 T-cell responses. During acute infections, IL-2 controls expansion and differentiation of antiviral CD8 T cells, whereas IL-7 and IL-15 are key cytokines to maintain memory CD8 T cells long term in an antigen-independent manner. On the other hand, during chronic infections, in which T-cell exhaustion is established, precise roles of these cytokines in regulation of antiviral CD8 T-cell responses are not well defined. Nonetheless, administration of IL-2, IL-7, or IL-15 can increase function of exhausted CD8 T cells, and thus can be an attractive therapeutic approach. A new subset of stem-cell-like CD8 T cells, which provides a proliferative burst after programmed cell death (PD)-1 therapy, has been recently described during chronic viral infection. Further understanding of cytokine-mediated regulation of this CD8 T-cell subset will improve cytokine therapies to treat chronic infections and cancer in combination with immune checkpoint inhibitors.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures

Similar articles
-
The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands.J Immunol. 2008 Nov 15;181(10):6738-46. doi: 10.4049/jimmunol.181.10.6738. J Immunol. 2008. PMID: 18981091
-
IL2Rβ-dependent signals drive terminal exhaustion and suppress memory development during chronic viral infection.Proc Natl Acad Sci U S A. 2016 Sep 13;113(37):E5444-53. doi: 10.1073/pnas.1604256113. Epub 2016 Aug 29. Proc Natl Acad Sci U S A. 2016. PMID: 27573835 Free PMC article.
-
Antigen-independent induction of Tim-3 expression on human T cells by the common γ-chain cytokines IL-2, IL-7, IL-15, and IL-21 is associated with proliferation and is dependent on the phosphoinositide 3-kinase pathway.J Immunol. 2012 Apr 15;188(8):3745-56. doi: 10.4049/jimmunol.1102609. Epub 2012 Mar 14. J Immunol. 2012. PMID: 22422881
-
CD8 T Cell Exhaustion in Chronic Infection and Cancer: Opportunities for Interventions.Annu Rev Med. 2018 Jan 29;69:301-318. doi: 10.1146/annurev-med-012017-043208. Annu Rev Med. 2018. PMID: 29414259 Review.
-
Gamma-Chain Receptor Cytokines & PD-1 Manipulation to Restore HCV-Specific CD8+ T Cell Response during Chronic Hepatitis C.Cells. 2021 Mar 3;10(3):538. doi: 10.3390/cells10030538. Cells. 2021. PMID: 33802622 Free PMC article. Review.
Cited by
-
Tumor-associated macrophages and CD8+ T cells: dual players in the pathogenesis of HBV-related HCC.Front Immunol. 2024 Oct 10;15:1472430. doi: 10.3389/fimmu.2024.1472430. eCollection 2024. Front Immunol. 2024. PMID: 39450177 Free PMC article. Review.
-
IL-27 promotes the expansion of self-renewing CD8+ T cells in persistent viral infection.J Exp Med. 2019 Aug 5;216(8):1791-1808. doi: 10.1084/jem.20190173. Epub 2019 Jun 4. J Exp Med. 2019. PMID: 31164392 Free PMC article.
-
Degenerate CD8 Epitopes Mapping to Structurally Constrained Regions of the Spike Protein: A T Cell-Based Way-Out From the SARS-CoV-2 Variants Storm.Front Immunol. 2021 Sep 8;12:730051. doi: 10.3389/fimmu.2021.730051. eCollection 2021. Front Immunol. 2021. PMID: 34566990 Free PMC article. Review.
-
Cytokine Adjuvants IL-7 and IL-15 Improve Humoral Responses of a SHIV LentiDNA Vaccine in Animal Models.Vaccines (Basel). 2022 Mar 17;10(3):461. doi: 10.3390/vaccines10030461. Vaccines (Basel). 2022. PMID: 35335093 Free PMC article.
-
HIV-1 trans-Infection Mediated by DCs: The Tip of the Iceberg of Cell-to-Cell Viral Transmission.Pathogens. 2021 Dec 31;11(1):39. doi: 10.3390/pathogens11010039. Pathogens. 2021. PMID: 35055987 Free PMC article. Review.
References
-
- Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. 1997. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell 89: 1033–1041. - PubMed
-
- Bachmann MF, Wolint P, Schwarz K, Jager P, Oxenius A. 2005. Functional properties and lineage relationship of CD8+ T cell subsets identified by expression of IL-7 receptor and CD62L. J Immunol 175: 4686–4696. - PubMed
-
- Bachmann MF, Beerli RR, Agnellini P, Wolint P, Schwarz K, Oxenius A. 2006. Long-lived memory CD8+ T cells are programmed by prolonged antigen exposure and low levels of cellular activation. Eur J Immunol 36: 842–854. - PubMed
-
- Bachmann MF, Wolint P, Walton S, Schwarz K, Oxenius A. 2007. Differential role of IL-2R signaling for CD8+ T cell responses in acute and chronic viral infections. Eur J Immunol 37: 1502–1512. - PubMed
-
- Barber DL, Wherry EJ, Ahmed R. 2003. Cutting edge: Rapid in vivo killing by memory CD8 T cells. J Immunol 171: 27–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
