Cytokines in Cancer Immunotherapy
- PMID: 29101107
- PMCID: PMC6280701
- DOI: 10.1101/cshperspect.a028472
Cytokines in Cancer Immunotherapy
Abstract
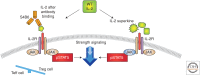
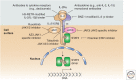
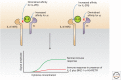
Cytokines that control the immune response were shown to have efficacy in preclinical murine cancer models. Interferon (IFN)-α is approved for treatment of hairy cell leukemia, and interleukin (IL)-2 for the treatment of advanced melanoma and metastatic renal cancer. In addition, IL-12, IL-15, IL-21, and granulocyte macrophage colony-stimulating factor (GM-CSF) have been evaluated in clinical trials. However, the cytokines as monotherapy have not fulfilled their early promise because cytokines administered parenterally do not achieve sufficient concentrations in the tumor, are often associated with severe toxicities, and induce humoral or cellular checkpoints. To circumvent these impediments, cytokines are being investigated clinically in combination therapy with checkpoint inhibitors, anticancer monoclonal antibodies to increase the antibody-dependent cellular cytotoxicity (ADCC) of these antibodies, antibody cytokine fusion proteins, and anti-CD40 to facilitate tumor-specific immune responses.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


References
-
- Alexander WS, Hilton DJ. 2004. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol 22: 503–529. - PubMed
-
- Amato R. 1999. Modest effect of interferon α on metastatic renal-cell carcinoma. Lancet 353: 6–7. - PubMed
-
- Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, Abrams J, Sznol M, Parkinson D, Hawkins M, et al. 1999. High-dose recombinant interleukin-2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol 17: 2105–2116. - PubMed
-
- Bamford RN, DeFilippis AP, Azimi N, Kurys G, Waldmann TA. 1998. The 5′ untranslated region, signal peptide and the coding sequence of the carboxyl terminus of IL-15 participate in its multifaceted translation control. J Immunol 160: 4418–4426. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
