Loss of caveolin-3-dependent regulation of ICa in rat ventricular myocytes in heart failure
- PMID: 29101175
- PMCID: PMC5899261
- DOI: 10.1152/ajpheart.00458.2017
Loss of caveolin-3-dependent regulation of ICa in rat ventricular myocytes in heart failure
Abstract
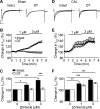
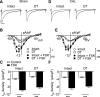
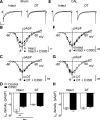
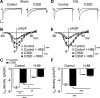
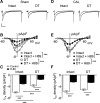
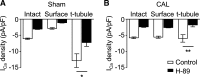
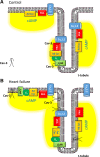
β2-Adrenoceptors and L-type Ca2+ current ( ICa) redistribute from the t-tubules to the surface membrane of ventricular myocytes from failing hearts. The present study investigated the role of changes in caveolin-3 and PKA signaling, both of which have previously been implicated in this redistribution. ICa was recorded using the whole cell patch-clamp technique from ventricular myocytes isolated from the hearts of rats that had undergone either coronary artery ligation (CAL) or equivalent sham operation 18 wk earlier. ICa distribution between the surface and t-tubule membranes was determined using formamide-induced detubulation (DT). In sham myocytes, β2-adrenoceptor stimulation increased ICa in intact but not DT myocytes; however, forskolin (to increase cAMP directly) and H-89 (to inhibit PKA) increased and decreased, respectively, ICa at both the surface and t-tubule membranes. C3SD peptide (which decreases binding to caveolin-3) inhibited ICa in intact but not DT myocytes but had no effect in the presence of H-89. In contrast, in CAL myocytes, β2-adrenoceptor stimulation increased ICa in both intact and DT myocytes, but C3SD had no effect on ICa; forskolin and H-89 had similar effects as in sham myocytes. These data show the redistribution of β2 -adrenoceptor activity and ICa in CAL myocytes and suggest constitutive stimulation of ICa by PKA in sham myocytes via concurrent caveolin-3-dependent (at the t-tubules) and caveolin-3-independent mechanisms, with the former being lost in CAL myocytes. NEW & NOTEWORTHY In ventricular myocytes from normal hearts, regulation of the L-type Ca2+ current by β2-adrenoceptors and the constitutive regulation by caveolin-3 is localized to the t-tubules. In heart failure, the regulation of L-type Ca2+ current by β2-adrenoceptors is redistributed to the surface membrane, and the constitutive regulation by caveolin-3 is lost.
Keywords: L-type Ca2+ current; cAMP; caveolin-3; heart failure; myocardial infarction.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

