Heart functional and structural compendium of cardiosplenic and cardiorenal networks in acute and chronic heart failure pathology
- PMID: 29101178
- PMCID: PMC5867656
- DOI: 10.1152/ajpheart.00528.2017
Heart functional and structural compendium of cardiosplenic and cardiorenal networks in acute and chronic heart failure pathology
Abstract
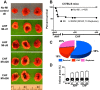
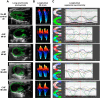
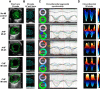
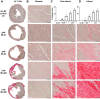
Heart failure (HF) secondary to myocardial infarction (MI) is linked to kidney complications that comprise cellular, structural, functional, and survival indicators. However, HF research is focused on left ventricular (LV) pathology. Here, we determined comprehensive functional analysis of the LV using echocardiography in transition from acute heart failure (AHF) to progressive chronic heart failure (CHF) pathology and developed a histological compendium of the cardiosplenic and cardiorenal networks in pathological remodeling. In surgically induced MI using permanent coronary ligation, the LV dysfunction is pronounced, with myocardium necrosis, wall thinning, and 20-30% LV rupture events that indicated AHF and CHF pathological remodeling in C57BL/6 male mice (2-4 mo old, n = 50). Temporal LV function analysis indicated that fractional shortening and strain are reduced from day 1 to day 5 in AHF and sustained to advance to CHF from day 28 to day 56 compared with naïve control mice ( n = 6). During the transition of AHF ( day 1 to day 5) to advanced CHF ( day 28 to day 56), histological and cellular changes in the spleen were definite, with bimodal inflammatory responses in kidney inflammatory biomarkers. Likewise, there was a unidirectional, progressive, and irreversible deposition of compact collagen in the LV along with dynamic changes in the cardiosplenic and cardiorenal networks post-MI. The renal histology and injury markers suggested that cardiac injury triggers irreversible dysregulation that actively alters the cardiosplenic and cardiorenal networks. In summary, the novel strategies or pathways that modulate comprehensive cardiosplenic and cardiorenal networks in AHF and CHF would be effective approaches to study either cardiac repair or cardiac pathology. NEW & NOTEWORTHY The present compendium shows irreversible ventricular dysfunction as assessed by temporal echocardiography while histological and structural measurements of the spleen and kidney added a novel direction to study cardiosplenic and cardiorenal networks in heart failure pathology. Therefore, the consideration of systems biology and integrative approach is essential to develop novel treatments.
Keywords: fibrosis; ischemia injury; temporal echocardiography.
Figures


Similar articles
-
Macro- and microinjury define the heart failure progression after permanent coronary ligation or ischemia-reperfusion in young healthy mice.Am J Physiol Heart Circ Physiol. 2025 Aug 1;329(2):H521-H533. doi: 10.1152/ajpheart.00267.2025. Epub 2025 Jul 16. Am J Physiol Heart Circ Physiol. 2025. PMID: 40668645
-
Clinical aspects of left ventricular diastolic function assessed by Doppler echocardiography following acute myocardial infarction.Dan Med Bull. 2001 Nov;48(4):199-210. Dan Med Bull. 2001. PMID: 11767125 Review.
-
Subtotal nephrectomy accelerates pathological cardiac remodeling post-myocardial infarction: implications for cardiorenal syndrome.Int J Cardiol. 2013 Oct 3;168(3):1866-80. doi: 10.1016/j.ijcard.2012.12.065. Epub 2013 Jan 22. Int J Cardiol. 2013. PMID: 23347614
-
[INTERRELATIONS BETWEEN MICROALBUMINURIA AND RENAL FUNCTION WITH CONTRACTILE ABILITY OF LEFT VENTRICLE IN CARDIORENAL SYNDROME PATIENTS].Georgian Med News. 2015 Oct;(247):34-8. Georgian Med News. 2015. PMID: 26483371 Russian.
-
Target organ cross talk in cardiorenal syndrome: animal models.Am J Physiol Renal Physiol. 2012 Nov 1;303(9):F1253-63. doi: 10.1152/ajprenal.00392.2012. Epub 2012 Aug 22. Am J Physiol Renal Physiol. 2012. PMID: 22914779 Review.
Cited by
-
Splenic leukocytes define the resolution of inflammation in heart failure.Sci Signal. 2018 Mar 6;11(520):eaao1818. doi: 10.1126/scisignal.aao1818. Sci Signal. 2018. PMID: 29511119 Free PMC article.
-
Activation of EP4 receptor limits transition of acute to chronic heart failure in lipoxygenase deficient mice.Theranostics. 2021 Jan 1;11(6):2742-2754. doi: 10.7150/thno.51183. eCollection 2021. Theranostics. 2021. PMID: 33456570 Free PMC article.
-
HIV Protein Nef Induces Cardiomyopathy Through Induction of Bcl2 and p21.Int J Mol Sci. 2024 Oct 23;25(21):11401. doi: 10.3390/ijms252111401. Int J Mol Sci. 2024. PMID: 39518954 Free PMC article.
-
Chronic Benzene Exposure Aggravates Pressure Overload-Induced Cardiac Dysfunction.Toxicol Sci. 2021 Dec 28;185(1):64-76. doi: 10.1093/toxsci/kfab125. Toxicol Sci. 2021. PMID: 34718823 Free PMC article.
-
Guidelines for experimental models of myocardial ischemia and infarction.Am J Physiol Heart Circ Physiol. 2018 Apr 1;314(4):H812-H838. doi: 10.1152/ajpheart.00335.2017. Epub 2018 Jan 12. Am J Physiol Heart Circ Physiol. 2018. PMID: 29351451 Free PMC article.
References
-
- Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol 63: 1123–1133, 2014. doi:10.1016/j.jacc.2013.11.053. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

