Ferroportin disease: pathogenesis, diagnosis and treatment
- PMID: 29101207
- PMCID: PMC5709096
- DOI: 10.3324/haematol.2017.170720
Ferroportin disease: pathogenesis, diagnosis and treatment
Abstract
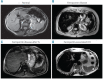
Ferroportin Disease (FD) is an autosomal dominant hereditary iron loading disorder associated with heterozygote mutations of the ferroportin-1 (FPN) gene. It represents one of the commonest causes of genetic hyperferritinemia, regardless of ethnicity. FPN1 transfers iron from the intestine, macrophages and placenta into the bloodstream. In FD, loss-of-function mutations of FPN1 limit but do not impair iron export in enterocytes, but they do severely affect iron transfer in macrophages. This leads to progressive and preferential iron trapping in tissue macrophages, reduced iron release to serum transferrin (i.e. inappropriately low transferrin saturation) and a tendency towards anemia at menarche or after intense bloodletting. The hallmark of FD is marked iron accumulation in hepatic Kupffer cells. Numerous FD-associated mutations have been reported worldwide, with a few occurring in different populations and some more commonly reported (e.g. Val192del, A77D, and G80S). FPN1 polymorphisms also represent the gene variants most commonly responsible for hyperferritinemia in Africans. Differential diagnosis includes mainly hereditary hemochromatosis, the syndrome commonly due to either HFE or TfR2, HJV, HAMP, and, in rare instances, FPN1 itself. Here, unlike FD, hyperferritinemia associates with high transferrin saturation, iron-spared macrophages, and progressive parenchymal cell iron load. Abdominal magnetic resonance imaging (MRI), the key non-invasive diagnostic tool for the diagnosis of FD, shows the characteristic iron loading SSL triad (spleen, spine and liver). A non-aggressive phlebotomy regimen is recommended, with careful monitoring of transferrin saturation and hemoglobin due to the risk of anemia. Family screening is mandatory since siblings and offspring have a 50% chance of carrying the pathogenic mutation.
Copyright© 2017 Ferrata Storti Foundation.
Figures




Similar articles
-
Hepatic expression of hemochromatosis genes in two mouse strains after phlebotomy and iron overload.Haematologica. 2005 Sep;90(9):1161-7. Haematologica. 2005. PMID: 16154838
-
Lack of enterocyte iron accumulation in the ferroportin disease.Blood Cells Mol Dis. 2005 Nov-Dec;35(3):315-8. doi: 10.1016/j.bcmd.2005.07.010. Epub 2005 Aug 30. Blood Cells Mol Dis. 2005. PMID: 16135412
-
The ferroportin disease.Blood Cells Mol Dis. 2004 Jan-Feb;32(1):131-8. doi: 10.1016/j.bcmd.2003.08.003. Blood Cells Mol Dis. 2004. PMID: 14757427 Review.
-
A Japanese family with ferroportin disease caused by a novel mutation of SLC40A1 gene: hyperferritinemia associated with a relatively low transferrin saturation of iron.Intern Med. 2005 Sep;44(9):990-3. doi: 10.2169/internalmedicine.44.990. Intern Med. 2005. PMID: 16258219
-
Non-HFE hepatic iron overload.Semin Liver Dis. 2011 Aug;31(3):302-18. doi: 10.1055/s-0031-1286061. Epub 2011 Sep 7. Semin Liver Dis. 2011. PMID: 21901660 Review.
Cited by
-
Nrf2 activators for the treatment of rare iron overload diseases: From bench to bedside.Redox Biol. 2025 Apr;81:103551. doi: 10.1016/j.redox.2025.103551. Epub 2025 Feb 14. Redox Biol. 2025. PMID: 39965404 Free PMC article. Review.
-
HFE-Related Hemochromatosis May Be a Primary Kupffer Cell Disease.Biomedicines. 2025 Mar 10;13(3):683. doi: 10.3390/biomedicines13030683. Biomedicines. 2025. PMID: 40149659 Free PMC article. Review.
-
The Importance of Iron Status for Young Children in Low- and Middle-Income Countries: A Narrative Review.Pharmaceuticals (Basel). 2019 Apr 16;12(2):59. doi: 10.3390/ph12020059. Pharmaceuticals (Basel). 2019. PMID: 30995720 Free PMC article. Review.
-
MRI-Based Iron Phenotyping and Patient Selection for Next-Generation Sequencing of Non-Homeostatic Iron Regulator Hemochromatosis Genes.Hepatology. 2021 Nov;74(5):2424-2435. doi: 10.1002/hep.31982. Epub 2021 Jul 13. Hepatology. 2021. PMID: 34048062 Free PMC article.
-
A Review of New Concepts in Iron Overload.Gastroenterol Hepatol (N Y). 2024 Feb;20(2):98-107. Gastroenterol Hepatol (N Y). 2024. PMID: 38414914 Free PMC article.
References
-
- Pietrangelo A. The ferroportin disease. Blood Cells Mol Dis. 2004;32(1):131–138. - PubMed
-
- Abboud S, Haile DJ. A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem. 2000;275(26):19906–19912. - PubMed
-
- Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403(6771):776–781. - PubMed
-
- McKie AT, Marciani P, Rolfs A, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5(2):299–309. - PubMed
-
- Njajou OT, Vaessen N, Joosse M, et al. A mutation in SLC11A3 is associated with autosomal dominant hemochromatosis. Nat Genet. 2001;28(3):213–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

