AlmG, responsible for polymyxin resistance in pandemic Vibrio cholerae, is a glycyltransferase distantly related to lipid A late acyltransferases
- PMID: 29101229
- PMCID: PMC5743092
- DOI: 10.1074/jbc.RA117.000131
AlmG, responsible for polymyxin resistance in pandemic Vibrio cholerae, is a glycyltransferase distantly related to lipid A late acyltransferases
Abstract
Cationic antimicrobial peptides (CAMPs), such as polymyxins, are used as a last-line defense in treatment of many bacterial infections. However, some bacteria have developed resistance mechanisms to survive these compounds. Current pandemic O1 Vibrio cholerae biotype El Tor is resistant to polymyxins, whereas a previous pandemic strain of the biotype Classical is polymyxin-sensitive. The almEFG operon found in El Tor V. cholerae confers >100-fold resistance to antimicrobial peptides through aminoacylation of lipopolysaccharide (LPS), expected to decrease the negatively charged surface of the V. cholerae outer membrane. This Gram-negative system bears striking resemblance to a related Gram-positive cell-wall remodeling strategy that also promotes CAMP resistance. Mutants defective in AlmEF-dependent LPS modification exhibit reduced fitness in vivo Here, we present investigation of AlmG, the hitherto uncharacterized member of the AlmEFG pathway. Evidence for AlmG glycyl to lipid substrate transferase activity is demonstrated in vivo by heterologous expression of V. cholerae pathway enzymes in a specially engineered Escherichia coli strain. Development of a minimal keto-deoxyoctulosonate (Kdo)-lipid A domain in E. coli was necessary to facilitate chemical structure analysis and to produce a mimetic Kdo-lipid A domain AlmG substrate to that synthesized by V. cholerae. Our biochemical studies support a uniquely nuanced pathway of Gram-negative CAMPs resistance and provide a more detailed description of an enzyme of the pharmacologically relevant lysophosphospholipid acyltransferase (LPLAT) superfamily.
Keywords: LABLAT; LPLAT; Vibrio cholera; acyltransferase; aminoacyltransferase; antibiotic resistance; antimicrobial peptide (AMP); bacterial membrane; bacterial pathogenesis; lipopolysaccharide.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


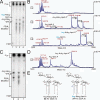




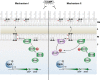
Comment in
-
Vibrio cholerae amino acids go on the defense.J Biol Chem. 2017 Dec 22;292(51):21216-21217. doi: 10.1074/jbc.H117.000868. J Biol Chem. 2017. PMID: 29273584 Free PMC article.
Similar articles
-
Amino acid addition to Vibrio cholerae LPS establishes a link between surface remodeling in gram-positive and gram-negative bacteria.Proc Natl Acad Sci U S A. 2012 May 29;109(22):8722-7. doi: 10.1073/pnas.1201313109. Epub 2012 May 15. Proc Natl Acad Sci U S A. 2012. PMID: 22589301 Free PMC article.
-
Polymyxin B resistance in El Tor Vibrio cholerae requires lipid acylation catalyzed by MsbB.J Bacteriol. 2010 Apr;192(8):2044-52. doi: 10.1128/JB.00023-10. Epub 2010 Feb 12. J Bacteriol. 2010. PMID: 20154134 Free PMC article.
-
Novel coordination of lipopolysaccharide modifications in Vibrio cholerae promotes CAMP resistance.Mol Microbiol. 2017 Nov;106(4):582-596. doi: 10.1111/mmi.13835. Epub 2017 Oct 6. Mol Microbiol. 2017. PMID: 28906060 Free PMC article.
-
Genetic and Biochemical Mechanisms for Bacterial Lipid A Modifiers Associated with Polymyxin Resistance.Trends Biochem Sci. 2019 Nov;44(11):973-988. doi: 10.1016/j.tibs.2019.06.002. Epub 2019 Jul 3. Trends Biochem Sci. 2019. PMID: 31279652 Review.
-
Mechanisms of Polymyxin Resistance.Adv Exp Med Biol. 2019;1145:55-71. doi: 10.1007/978-3-030-16373-0_5. Adv Exp Med Biol. 2019. PMID: 31364071 Review.
Cited by
-
A Whole-Cell Screen Identifies Small Bioactives That Synergize with Polymyxin and Exhibit Antimicrobial Activities against Multidrug-Resistant Bacteria.Antimicrob Agents Chemother. 2020 Feb 21;64(3):e01677-19. doi: 10.1128/AAC.01677-19. Print 2020 Feb 21. Antimicrob Agents Chemother. 2020. PMID: 31844003 Free PMC article.
-
A Periplasmic Antimicrobial Peptide-Binding Protein Is Required for Stress Survival in Vibrio cholerae.Front Microbiol. 2019 Feb 5;10:161. doi: 10.3389/fmicb.2019.00161. eCollection 2019. Front Microbiol. 2019. PMID: 30804918 Free PMC article.
-
Categorizing Sequences of Concern by Function To Better Assess Mechanisms of Microbial Pathogenesis.Infect Immun. 2022 May 19;90(5):e0033421. doi: 10.1128/IAI.00334-21. Epub 2021 Nov 15. Infect Immun. 2022. PMID: 34780277 Free PMC article. Review.
-
Public health aspects of Vibrio spp. related to the consumption of seafood in the EU.EFSA J. 2024 Jul 23;22(7):e8896. doi: 10.2903/j.efsa.2024.8896. eCollection 2024 Jul. EFSA J. 2024. PMID: 39045511 Free PMC article.
-
Genetic and Phenotypic Virulence Potential of Non-O1/Non-O139 Vibrio cholerae Isolated from German Retail Seafood.Microorganisms. 2023 Nov 11;11(11):2751. doi: 10.3390/microorganisms11112751. Microorganisms. 2023. PMID: 38004762 Free PMC article.
References
-
- Oger P. M. (2015) Homeoviscous adaptation of membranes in archaea. Subcell. Biochem. 72, 383–403 - PubMed
-
- Zhang Y.-M., and Rock C. O. (2008) Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 6, 222–233 - PubMed
-
- Ernst R., Ejsing C. S., and Antonny B. (2016) Homeoviscous adaptation and the regulation of membrane lipids. J. Mol. Biol. 428, 4776–4791 - PubMed
-
- Clementz T., Bednarski J. J., and Raetz C. R. (1996) Function of the htrB high temperature gene of Escherichia coli in the acylation of lipid A. J. Biol. Chem. 271, 12095–12102 - PubMed
-
- Vorachek-Warren M. K., Carty S. M., Lin S., Cotter R. J., and Raetz C. R. (2002) An Escherichia coli mutant lacking the cold shock-induced palmitoleoyltransferase of lipid A biosynthesis. Absence of unsaturated acyl chains and antibiotic hypersensitivity at 12 °C. J. Biol. Chem. 277, 14186–14193 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

