Abrogated Freud-1/Cc2d1a Repression of 5-HT1A Autoreceptors Induces Fluoxetine-Resistant Anxiety/Depression-Like Behavior
- PMID: 29101244
- PMCID: PMC5722640
- DOI: 10.1523/JNEUROSCI.1668-17.2017
Abrogated Freud-1/Cc2d1a Repression of 5-HT1A Autoreceptors Induces Fluoxetine-Resistant Anxiety/Depression-Like Behavior
Abstract
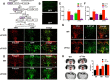
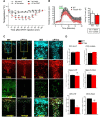
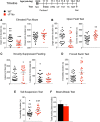
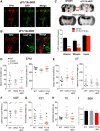
Freud-1/Cc2d1a represses the gene transcription of serotonin-1A (5-HT1A) autoreceptors, which negatively regulate 5-HT tone. To test the role of Freud-1 in vivo, we generated mice with adulthood conditional knock-out of Freud-1 in 5-HT neurons (cF1ko). In cF1ko mice, 5-HT1A autoreceptor protein, binding and hypothermia response were increased, with reduced 5-HT content and neuronal activity in the dorsal raphe. The cF1ko mice displayed increased anxiety- and depression-like behavior that was resistant to chronic antidepressant (fluoxetine) treatment. Using conditional Freud-1/5-HT1A double knock-out (cF1/1A dko) to disrupt both Freud-1 and 5-HT1A genes in 5-HT neurons, no increase in anxiety- or depression-like behavior was seen upon knock-out of Freud-1 on the 5-HT1A autoreceptor-negative background; rather, a reduction in depression-like behavior emerged. These studies implicate transcriptional dysregulation of 5-HT1A autoreceptors by the repressor Freud-1 in anxiety and depression and provide a clinically relevant genetic model of antidepressant resistance. Targeting specific transcription factors, such as Freud-1, to restore transcriptional balance may augment response to antidepressant treatment.SIGNIFICANCE STATEMENT Altered regulation of the 5-HT1A autoreceptor has been implicated in human anxiety, major depression, suicide, and resistance to antidepressants. This study uniquely identifies a single transcription factor, Freud-1, as crucial for 5-HT1A autoreceptor expression in vivo Disruption of Freud-1 in serotonin neurons in mice links upregulation of 5-HT1A autoreceptors to anxiety/depression-like behavior and provides a new model of antidepressant resistance. Treatment strategies to reestablish transcriptional regulation of 5-HT1A autoreceptors could provide a more robust and sustained antidepressant response.
Keywords: 5-HT1A receptor; anxiety; major depression; raphe; repressor; serotonin.
Copyright © 2017 the authors 0270-6474/17/3711967-12$15.00/0.
Figures

References
-
- Albert PR, Zhou QY, Van Tol HH, Bunzow JR, Civelli O (1990) Cloning, functional expression, and mRNA tissue distribution of the rat 5-hydroxytryptamine1A receptor gene. J Biol Chem 265:5825–5832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
