Plasmodium DNA-mediated TLR9 activation of T-bet+ B cells contributes to autoimmune anaemia during malaria
- PMID: 29101363
- PMCID: PMC5670202
- DOI: 10.1038/s41467-017-01476-6
Plasmodium DNA-mediated TLR9 activation of T-bet+ B cells contributes to autoimmune anaemia during malaria
Abstract
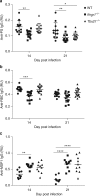
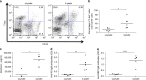
Infectious pathogens contribute to the development of autoimmune disorders, but the mechanisms connecting these processes are incompletely understood. Here we show that Plasmodium DNA induces autoreactive responses against erythrocytes by activating a population of B cells expressing CD11c and the transcription factor T-bet, which become major producers of autoantibodies that promote malarial anaemia. Additionally, we identify parasite DNA-sensing through Toll-like receptor 9 (TLR9) along with inflammatory cytokine receptor IFN-γ receptor (IFN-γR) as essential signals that synergize to promote the development and appearance of these autoreactive T-bet+ B cells. The lack of any of these signals ameliorates malarial anaemia during infection in a mouse model. We also identify both expansion of T-bet+ B cells and production of anti-erythrocyte antibodies in ex vivo cultures of naive human peripheral blood mononuclear cells (PBMC) exposed to P. falciprum infected erythrocyte lysates. We propose that synergistic TLR9/IFN-γR activation of T-bet+ B cells is a mechanism underlying infection-induced autoimmune-like responses.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






References
-
- Rivera-Correa, J. R. A. in Malaria: Immune Response to Infection and Vaccination (eds Rodriguez, A. & Mota, M.) (Springer, Switzerland, 2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

