Keeping Neurons Young and Foxy: FoxOs Promote Neuronal Plasticity
- PMID: 29102406
- PMCID: PMC5741477
- DOI: 10.1016/j.tig.2017.10.002
Keeping Neurons Young and Foxy: FoxOs Promote Neuronal Plasticity
Abstract
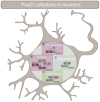
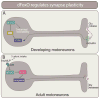
Any adult who has tried to take up the piano or learn a new language is faced with the sobering realization that acquiring such skills is more challenging as an adult than as a child. Neuronal plasticity, or the malleability of brain circuits, declines with age. Young neurons tend to be more adaptable and can alter the size and strength of their connections more readily than can old neurons. Myriad circuit- and synapse-level mechanisms that shape plasticity have been identified. Yet, molecular mechanisms setting the overall competence of young neurons for distinct forms of plasticity remain largely obscure. Recent studies indicate evolutionarily conserved roles for FoxO proteins in establishing the capacity for cell-fate, morphological, and synaptic plasticity in neurons.
Keywords: Daf-16; FoxO; microtubule dynamics; neurodegeneration; neuronal plasticity; synaptic plasticity.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


References
-
- Kenyon C, et al. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. - PubMed
-
- Lin K, et al. daf-16: An HNF-3/forkhead family member that can function to double the life-span of Caenorhabditis elegans. Science. 1997;278:1319–1322. - PubMed
-
- Ogg S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

