Prostaglandin E2 Increases Lentiviral Vector Transduction Efficiency of Adult Human Hematopoietic Stem and Progenitor Cells
- PMID: 29102562
- PMCID: PMC5763075
- DOI: 10.1016/j.ymthe.2017.09.025
Prostaglandin E2 Increases Lentiviral Vector Transduction Efficiency of Adult Human Hematopoietic Stem and Progenitor Cells
Abstract
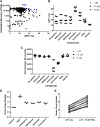
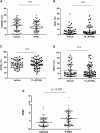
Gene therapy currently in development for hemoglobinopathies utilizes ex vivo lentiviral transduction of CD34+ hematopoietic stem and progenitor cells (HSPCs). A small-molecule screen identified prostaglandin E2 (PGE2) as a positive mediator of lentiviral transduction of CD34+ cells. Supplementation with PGE2 increased lentiviral vector (LVV) transduction of CD34+ cells approximately 2-fold compared to control transduction methods with no effect on cell viability. Transduction efficiency was consistently increased in primary CD34+ cells from multiple normal human donors and from patients with β-thalassemia or sickle cell disease. Notably, PGE2 increased transduction of repopulating human HSPCs in an immune-deficient (nonobese diabetic/severe combined immunodeficiency/interleukin-2 gamma receptor null [NSG]) xenotransplantation mouse model without evidence of in vivo toxicity, lineage bias, or a de novo bias of lentiviral integration sites. These data suggest that PGE2 improves lentiviral transduction and increases vector copy number, therefore resulting in increased transgene expression. As a result, PGE2 may be useful in clinical gene therapy applications using lentivirally modified HSPCs.
Keywords: gene therapy; hematopoietic stem cell; hemoglobinopathy; lentiviral vector; prostaglandin E(2); transduction; vector copy number.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. - PubMed
-
- Scaramuzza S., Biasco L., Ripamonti A., Castiello M.C., Loperfido M., Draghici E., Hernandez R.J., Benedicenti F., Radrizzani M., Salomoni M. Preclinical safety and efficacy of human CD34(+) cells transduced with lentiviral vector for the treatment of Wiskott-Aldrich syndrome. Mol. Ther. 2013;21:175–184. - PMC - PubMed
-
- Sessa M., Lorioli L., Fumagalli F., Acquati S., Redaelli D., Baldoli C., Canale S., Lopez I.D., Morena F., Calabria A. Lentiviral haemopoietic stem-cell gene therapy in early-onset metachromatic leukodystrophy: an ad-hoc analysis of a non-randomised, open-label, phase 1/2 trial. Lancet. 2016;388:476–487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

