And yet it moves: Recovery of volitional control after spinal cord injury
- PMID: 29102670
- PMCID: PMC5773077
- DOI: 10.1016/j.pneurobio.2017.10.004
And yet it moves: Recovery of volitional control after spinal cord injury
Abstract
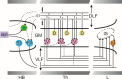
Preclinical and clinical neurophysiological and neurorehabilitation research has generated rather surprising levels of recovery of volitional sensory-motor function in persons with chronic motor paralysis following a spinal cord injury. The key factor in this recovery is largely activity-dependent plasticity of spinal and supraspinal networks. This key factor can be triggered by neuromodulation of these networks with electrical and pharmacological interventions. This review addresses some of the systems-level physiological mechanisms that might explain the effects of electrical modulation and how repetitive training facilitates the recovery of volitional motor control. In particular, we substantiate the hypotheses that: (1) in the majority of spinal lesions, a critical number and type of neurons in the region of the injury survive, but cannot conduct action potentials, and thus are electrically non-responsive; (2) these neuronal networks within the lesioned area can be neuromodulated to a transformed state of electrical competency; (3) these two factors enable the potential for extensive activity-dependent reorganization of neuronal networks in the spinal cord and brain, and (4) propriospinal networks play a critical role in driving this activity-dependent reorganization after injury. Real-time proprioceptive input to spinal networks provides the template for reorganization of spinal networks that play a leading role in the level of coordination of motor pools required to perform a given functional task. Repetitive exposure of multi-segmental sensory-motor networks to the dynamics of task-specific sensory input as occurs with repetitive training can functionally reshape spinal and supraspinal connectivity thus re-enabling one to perform complex motor tasks, even years post injury.
Keywords: Electrical stimulation; Motor training; Neuromodulation; Spinal networks.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Alford S., Schwartz E., Viana di Prisco G. The pharmacology of vertebrate spinal central pattern generators. Neuroscientist. 2003;9:217–228. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

