Melt extrusion with poorly soluble drugs - An integrated review
- PMID: 29102700
- PMCID: PMC5756511
- DOI: 10.1016/j.ijpharm.2017.10.056
Melt extrusion with poorly soluble drugs - An integrated review
Abstract
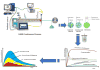
Over the last few decades, hot melt extrusion (HME) has emerged as a successful technology for a broad spectrum of applications in the pharmaceutical industry. As indicated by multiple publications and patents, HME is mainly used for the enhancement of solubility and bioavailability of poorly soluble drugs. This review is focused on the recent reports on the solubility enhancement via HME and provides an update for the manufacturing/scaling up aspects of melt extrusion. In addition, drug characterization methods and dissolution studies are discussed. The application of process analytical technology (PAT) tools and use of HME as a continuous manufacturing process may shorten the drug development process; as a result, the latter is becoming the most widely utilized technique in the pharmaceutical industry. The advantages, disadvantages, and practical applications of various PAT tools such as near and mid-infrared, ultraviolet/visible, fluorescence, and Raman spectroscopies are summarized, and the characteristics of other techniques are briefly discussed. Overall, this review also provides an outline for the currently marketed products and analyzes the strengths, weaknesses, opportunities and threats of HME application in the pharmaceutical industry.
Keywords: Continuous manufacturing; Dissolution; Hot melt extrusion; Polymer; Poorly soluble drug; Process analytical technology tool; SWOT analysis.
Published by Elsevier B.V.
Conflict of interest statement
The authors report no potential conflicts of interest.
Figures
Similar articles
-
An update on the contribution of hot-melt extrusion technology to novel drug delivery in the twenty-first century: part I.Expert Opin Drug Deliv. 2019 May;16(5):539-550. doi: 10.1080/17425247.2019.1609448. Epub 2019 May 3. Expert Opin Drug Deliv. 2019. PMID: 31007090 Free PMC article. Review.
-
Hot Melt Extruded and Injection Moulded Dosage Forms: Recent Research and Patents.Recent Pat Drug Deliv Formul. 2015;9(3):194-200. doi: 10.2174/1872211309666150512111143. Recent Pat Drug Deliv Formul. 2015. PMID: 27165308 Review.
-
Hot-Melt Extrusion: from Theory to Application in Pharmaceutical Formulation.AAPS PharmSciTech. 2016 Feb;17(1):20-42. doi: 10.1208/s12249-015-0360-7. Epub 2015 Jul 10. AAPS PharmSciTech. 2016. PMID: 26159653 Free PMC article. Review.
-
Hot-Melt Extrusion: An Emerging Technique for Solubility Enhancement of Poorly Water-Soluble Drugs.PDA J Pharm Sci Technol. 2021 Jul-Aug;75(4):357-373. doi: 10.5731/pdajpst.2019.011403. Epub 2021 Feb 19. PDA J Pharm Sci Technol. 2021. PMID: 33608469 Review.
-
Hot-melt extrusion technology and pharmaceutical application.Ther Deliv. 2012 Jun;3(6):787-97. doi: 10.4155/tde.12.26. Ther Deliv. 2012. PMID: 22838073 Review.
Cited by
-
Practicality of 3D Printed Personalized Medicines in Therapeutics.Front Pharmacol. 2021 Apr 12;12:646836. doi: 10.3389/fphar.2021.646836. eCollection 2021. Front Pharmacol. 2021. PMID: 33912058 Free PMC article. Review.
-
Novel On-Demand 3-Dimensional (3-D) Printed Tablets Using Fill Density as an Effective Release-Controlling Tool.Polymers (Basel). 2020 Aug 20;12(9):1872. doi: 10.3390/polym12091872. Polymers (Basel). 2020. PMID: 32825229 Free PMC article.
-
In-Line UV-Vis Spectroscopy as a Fast-Working Process Analytical Technology (PAT) during Early Phase Product Development Using Hot Melt Extrusion (HME).Pharmaceutics. 2018 Sep 23;10(4):166. doi: 10.3390/pharmaceutics10040166. Pharmaceutics. 2018. PMID: 30249025 Free PMC article.
-
Clay-Polymer Nanocomposites Prepared by Reactive Melt Extrusion for Sustained Drug Release.Pharmaceutics. 2020 Jan 7;12(1):51. doi: 10.3390/pharmaceutics12010051. Pharmaceutics. 2020. PMID: 31936176 Free PMC article.
-
Understanding the Interaction of Thermal, Rheological, and Mechanical Parameters Critical for the Processability of Polyvinyl Alcohol-Based Systems during Hot Melt Extrusion.Pharmaceutics. 2024 Mar 28;16(4):472. doi: 10.3390/pharmaceutics16040472. Pharmaceutics. 2024. PMID: 38675133 Free PMC article.
References
-
- Adler C, Schönenberger M, Teleki A, Kuentz M. Molecularly designed lipid microdomains for solid dispersions using a polymer/inorganic carrier matrix produced by hot-melt extrusion. Int J Pharm. 2016;499:90–100. - PubMed
-
- Ahmed HA, Alfredson TV, Birudaraj K, Brandl MT, Phuapradit W, Shah NH, Stefanidis D. Pharmaceutical composition and process. Google Patents 2010
-
- Alam MA, Ali R, Al-Jenoobi FI, Al-Mohizea AM. Solid dispersions: a strategy for poorly aqueous soluble drugs and technology updates. Expert Opin Drug Deliv. 2012;9:1419–1440. - PubMed
-
- Almeida A, Possemiers S, Boone M, De Beer T, Quinten T, Van Hoorebeke L, Remon JP, Vervaet C. Ethylene vinyl acetate as matrix for oral sustained release dosage forms produced via hot-melt extrusion. Eur J Pharm Biopharm. 2011;77:297–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials