A multi-dataset time-reversal approach to clinical trial placebo response and the relationship to natural variability in epilepsy
- PMID: 29102709
- PMCID: PMC5722663
- DOI: 10.1016/j.seizure.2017.10.016
A multi-dataset time-reversal approach to clinical trial placebo response and the relationship to natural variability in epilepsy
Abstract
Purpose: Clinical epilepsy drug trials have been measuring increasingly high placebo response rates, up to 40%. This study was designed to examine the relationship between the natural variability in epilepsy, and the placebo response seen in trials. We tested the hypothesis that 'reversing' trial direction, with the baseline period as the treatment observation phase, would reveal effects of natural variability.
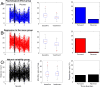
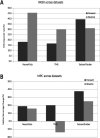
Method: Clinical trial simulations were run with time running forward and in reverse. Data sources were: SeizureTracker.com (patient reported diaries), a randomized sham-controlled TMS trial, and chronically implanted intracranial EEG electrodes. Outcomes were 50%-responder rates (RR50) and median percentage change (MPC).
Results: The RR50 results showed evidence that temporal reversal does not prevent large responder rates across datasets. The MPC results negative in the TMS dataset, and positive in the other two.
Conclusions: Typical RR50s of clinical trials can be reproduced using the natural variability of epilepsy as a substrate across multiple datasets. Therefore, the placebo response in epilepsy clinical trials may be attributable almost entirely to this variability, rather than the "placebo effect".
Keywords: Big data; Placebo; Placebo effect; Randomized clinical trial; Seizure diary; Simulation; Statistics.
Published by Elsevier Ltd.
Conflict of interest statement
None of the authors has any conflict of interest to disclose.
Figures

References
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. - PubMed
-
- Institute of Medicine (U.S.), Committee on the Public Health Dimensions of the Epilepsies, England MJ. Washington, DC: National Academies Press; 2012. [accessed Aug 28, 2014]. Epilepsy across the spectrum promoting health and understanding. http://www.nap.edu/catalog.php?record_id=13379. - PubMed
-
- PhRMA. Profile Biopharmaceutical Research Industry. 2015 http://www.phrma.org/sites/default/files/pdf/2015_phrma_profile.pdf.
-
- Rheims S, Perucca E, Cucherat M, et al. Factors determining response to antiepileptic drugs in randomized controlled trials. A systematic review and meta-analysis: Response to AEDs in Randomized Trials. Epilepsia. 2011 no-no. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

