Stepwise dose increase of febuxostat is comparable with colchicine prophylaxis for the prevention of gout flares during the initial phase of urate-lowering therapy: results from FORTUNE-1, a prospective, multicentre randomised study
- PMID: 29102957
- PMCID: PMC5867413
- DOI: 10.1136/annrheumdis-2017-211574
Stepwise dose increase of febuxostat is comparable with colchicine prophylaxis for the prevention of gout flares during the initial phase of urate-lowering therapy: results from FORTUNE-1, a prospective, multicentre randomised study
Abstract
Objectives: To determine whether febuxostat with stepwise dose increase is as useful as colchicine prophylaxis in reducing gout flares during the initial introduction of urate-lowering therapy in patients with gout in comparison with febuxostat with no dose titration.
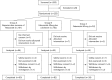
Methods: In this prospective, multicentre, randomised open-label comparative study, patients were randomised to group A (stepwise dose increase of febuxostat from 10 to 40 mg/day), group B (fixed-dose febuxostat 40 mg/day plus colchicine 0.5 mg/day) or group C (fixed-dose febuxostat 40 mg/day) and observed for 12 weeks. Gout flare was defined as non-steroidal anti-inflammatory drug use for gout symptoms.
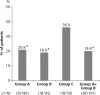
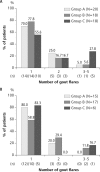
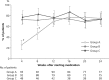
Results: A total of 255 patients were randomised, and 241 patients were treated. Among the treated patients, gout flares were experienced by 20/96 (20.8%) in group A, 18/95 (18.9%) in group B and 18/50 (36.0%) in group C. The incidence of flare was significantly lower in groups A and B than that in group C (P=0.047 and P=0.024, respectively), although the differences were not significant after correction for multiple comparisons. No significant difference was noted between the incidence of gout flare in groups A and B.
Conclusions: Our data suggested that stepwise dose increase of febuxostat and low-dose colchicine prophylaxis effectively reduced gout flares in comparison with fixed-dose febuxostat alone. Stepwise dose increase of febuxostat may be an effective alternative to low-dose colchicine prophylaxis during the introduction of urate-lowering therapy.
Trial registration number: UMIN 000008414.
Keywords: arthritis; gout; treatment.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2018. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: HY reports grants and personal fees from Teijin Pharma, Pfizer Japan, Takeda Pharmaceutical Company, Bristol-Myers Squibb, Nippon Kayaku, Chugai Pharmaceutical, Mitsubishi Tanabe Pharma, Daiichi Sankyo, Astellas Pharma, AbbVie, UCB Japan, Ono Pharmaceutical, Ayumi Pharmaceutical, Eisai, Torii Pharmaceutical, Taisho Toyama Pharmaceutical and YL Biologics, and grants from MSD. ST reports personal fees from Teijin Pharma. YH reports personal fees from Teijin Pharma, Pfizer Japan, Sanwa Kagaku Kenkyusho and Fujiyakuhin. AT reports personal fees from Teijin Pharma. SF reports personal fees from Teijin Pharma, Sanwa Kagaku Kenkyusho, Fujiyakuhin, Astellas Pharma, Kissei Pharmaceutical, Pfizer Japan, MSD and Nippon Chemiphar. TY reports personal fees and non-financial support from Teijin Pharma.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

