Randomized, Controlled Trial of TRC101 to Increase Serum Bicarbonate in Patients with CKD
- PMID: 29102959
- PMCID: PMC5753317
- DOI: 10.2215/CJN.07300717
Randomized, Controlled Trial of TRC101 to Increase Serum Bicarbonate in Patients with CKD
Erratum in
-
Correction.Clin J Am Soc Nephrol. 2019 Feb 7;14(2):277. doi: 10.2215/CJN.13671118. Epub 2019 Jan 10. Clin J Am Soc Nephrol. 2019. PMID: 30630860 Free PMC article. No abstract available.
Abstract
Background and objectives: Metabolic acidosis is common in patients with CKD and has significant adverse effects on kidney, muscle, and bone. We tested the efficacy and safety of TRC101, a novel, sodium-free, nonabsorbed hydrochloric acid binder, to increase serum bicarbonate in patients with CKD and metabolic acidosis.
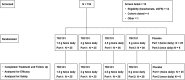
Design, setting, participants, & measurements: One hundred thirty-five patients were enrolled in this randomized, double-blind, placebo-controlled, multicenter, in-unit study (designated the TRCA-101 Study). Patients had a mean baseline eGFR of 35 ml/min per 1.73 m2, a mean baseline serum bicarbonate of 17.7 mEq/L, and comorbidities, including hypertension (93%), diabetes (70%), and heart failure (21%). Patients ate a controlled diet and were treated for 14 days with placebo or one of four TRC101 dosing regimens (1.5, 3, or 4.5 g twice daily or 6 g once daily). After treatment, patients were discharged and followed for 7-14 days.
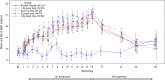
Results: All TRC101 treatment groups had a mean within-group increase in serum bicarbonate of ≥1.3 mEq/L (P<0.001) within 72 hours of the first dose and a mean increase in serum bicarbonate of 3.2-3.9 mEq/L (P<0.001) at the end of treatment compared with placebo, in which serum bicarbonate did not change. In the combined TRC101 treatment group, serum bicarbonate was normalized (22-29 mEq/L) at the end of treatment in 35% of patients and increased by ≥4 mEq/L in 39% of patients. After discontinuation of TRC101, serum bicarbonate decreased nearly to baseline levels within 2 weeks. All adverse events were mild or moderate, with gastrointestinal events most common. All patients completed the study.
Conclusions: TRC101 safely and significantly increased the level of serum bicarbonate in patients with metabolic acidosis and CKD.
Keywords: Diabetes Mellitus; Double-Blind Method; Heart Failure; Hydrochloric Acid; Renal Insufficiency, Chronic; acidosis; bicarbonates; chronic kidney disease; comorbidity; diet; humans; hypertension; kidney; metabolic acidosis, chronic; sodium.
Copyright © 2018 by the American Society of Nephrology.
Figures


Comment in
-
New Frontiers in Treating Uremic Metabolic Acidosis.Clin J Am Soc Nephrol. 2018 Jan 6;13(1):4-5. doi: 10.2215/CJN.11771017. Epub 2017 Nov 4. Clin J Am Soc Nephrol. 2018. PMID: 29102960 Free PMC article. No abstract available.
References
-
- Alpern RJ, Sakhaee K: The clinical spectrum of chronic metabolic acidosis: Homeostatic mechanisms produce significant morbidity. Am J Kidney Dis 29: 291–302, 1997 - PubMed
-
- Cohen RM, Feldman GM, Fernandez PC: The balance of acid, base and charge in health and disease. Kidney Int 52: 287–293, 1997 - PubMed
-
- Lemann J Jr, Bushinsky DA, Hamm LL: Bone buffering of acid and base in humans. Am J Physiol Renal Physiol 285: F811–F832, 2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

