Clinical and economic evaluation of modulated electrohyperthermia concurrent to dose-dense temozolomide 21/28 days regimen in the treatment of recurrent glioblastoma: a retrospective analysis of a two-centre German cohort trial with systematic comparison and effect-to-treatment analysis
- PMID: 29102988
- PMCID: PMC5722101
- DOI: 10.1136/bmjopen-2017-017387
Clinical and economic evaluation of modulated electrohyperthermia concurrent to dose-dense temozolomide 21/28 days regimen in the treatment of recurrent glioblastoma: a retrospective analysis of a two-centre German cohort trial with systematic comparison and effect-to-treatment analysis
Abstract
Objective: To assess the efficacy and cost-effectiveness of modulated electrohyperthermia (mEHT) concurrent to dose-dense temozolomide (ddTMZ) 21/28 days regimen versus ddTMZ 21/28 days alone in patients with recurrent glioblastoma (GBM).
Design: A cohort of 54 patients with recurrent GBM treated with ddTMZ+mEHT in 2000-2005 was systematically retrospectively compared with five pooled ddTMZ 21/28 days cohorts (114 patients) enrolled in 2008-2013.
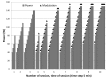
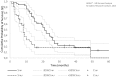
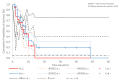
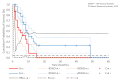
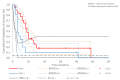
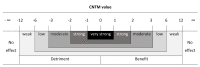
Results: The ddTMZ+mEHT cohort had a not significantly improved mean survival time (mST) versus the comparator (p=0.531) after a significantly less mean number of cycles (1.56 vs 3.98, p<0.001). Effect-to-treatment analysis (ETA) suggests that mEHT significantly enhances the efficacy of the ddTMZ 21/28 days regimen (p=0.011), with significantly less toxicity (no grade III-IV toxicity vs 45%-92%, p<0.0001). An estimated maximal attainable median survival time is 10.10 months (9.10-11.10). Cost-effectiveness analysis suggests that, unlike ddTMZ 21/28 days alone, ddTMZ+mEHT is cost-effective versus the applicable cost-effectiveness thresholds €US$25 000-50 000/quality-adjusted life year (QALY). Budget impact analysis suggests a significant saving of €8 577 947/$11 201 761 with 29.1-38.5 QALY gained per 1000 patients per year. Cost-benefit analysis suggests that mEHT is profitable and will generate revenues between €3 124 574 and $6 458 400, with a total economic effect (saving+revenues) of €5 700 034 to $8 237 432 per mEHT device over an 8-year period.
Conclusions: Our ETA suggests that mEHT significantly improves survival of patients receiving the ddTMZ 21/28 days regimen. Economic evaluation suggests that ddTMZ+mEHT is cost-effective, budget-saving and profitable. After confirmation of the results, mEHT could be recommended for the treatment of recurrent GBM as a cost-effective enhancer of ddTMZ regimens, and, probably, of the regular 5/28 days regimen. mEHT is applicable also as a single treatment if chemotherapy is impossible, and as a salvage treatment after the failure of chemotherapy.
Keywords: cost-effectiveness analysis; dose-dense temozolamide (ddtmz; effect-to-treatment analysis (eta); modulated electro-hyperthermia (meht); oncothermia; recurrent glioblastoma.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: None declared.
Figures


References
-
- Brandes AA, Fiorentino MV. The role of chemotherapy in recurrent malignant gliomas: an overview. Cancer Invest 1996;14:551–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
