Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3
- PMID: 29103793
- PMCID: PMC11366072
- DOI: 10.1016/j.ophtha.2017.09.028
Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3
Erratum in
-
Corrigendum.Ophthalmology. 2019 Jan;126(1):177. doi: 10.1016/j.ophtha.2018.10.038. Ophthalmology. 2019. PMID: 30577915 No abstract available.
Abstract
Purpose: To develop consensus terminology and criteria for defining atrophy based on OCT findings in the setting of age-related macular degeneration (AMD).
Design: Consensus meeting.
Participants: Panel of retina specialists, image reading center experts, retinal histologists, and optics engineers.
Methods: As part of the Classification of Atrophy Meetings (CAM) program, an international group of experts surveyed the existing literature, performed a masked analysis of longitudinal multimodal imaging for a series of eyes with AMD, and reviewed the results of this analysis to define areas of agreement and disagreement. Through consensus discussions at 3 meetings over 12 months, a classification system based on OCT was proposed for atrophy secondary to AMD. Specific criteria were defined to establish the presence of atrophy.
Main outcome measures: A consensus classification system for atrophy and OCT-based criteria to identify atrophy.
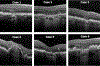
Results: OCT was proposed as the reference standard or base imaging method to diagnose and stage atrophy. Other methods, including fundus autofluorescence, near-infrared reflectance, and color imaging, provided complementary and confirmatory information. Recognizing that photoreceptor atrophy can occur without retinal pigment epithelium (RPE) atrophy and that atrophy can undergo an evolution of different stages, 4 terms and histologic candidates were proposed: complete RPE and outer retinal atrophy (cRORA), incomplete RPE and outer retinal atrophy, complete outer retinal atrophy, and incomplete outer retinal atrophy. Specific OCT criteria to diagnose cRORA were proposed: (1) a region of hypertransmission of at least 250 μm in diameter, (2) a zone of attenuation or disruption of the RPE of at least 250 μm in diameter, (3) evidence of overlying photoreceptor degeneration, and (4) absence of scrolled RPE or other signs of an RPE tear.
Conclusions: A classification system and criteria for OCT-defined atrophy in the setting of AMD has been proposed based on an international consensus. This classification is a more complete representation of changes that occur in AMD than can be detected using color fundus photography alone. Longitudinal information is required to validate the implied risk of vision loss associated with these terms. This system will enable such future studies to be undertaken using consistent definitions.
Copyright © 2017 American Academy of Ophthalmology. All rights reserved.
Figures




References
-
- Holz FG, Strauss EC, Schmitz-Valckenberg S, van Lookeren Campagne M. Geographic atrophy: clinical features and potential therapeutic approaches. Ophthalmology. 2014;121:1079–1091. - PubMed
-
- Gass JD. Stereoscopic Atlas of Macular Diseases. 1st ed. St. Louis: The C. V. Mosby Company; 1970.
-
- Haab O Erkrankungen der Macula Lutea. Centralblat Augenheilkd. 1885;9:384–391.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

