Local Order within Global Disorder: Synaptic Architecture of Visual Space
- PMID: 29103806
- PMCID: PMC5868972
- DOI: 10.1016/j.neuron.2017.10.017
Local Order within Global Disorder: Synaptic Architecture of Visual Space
Abstract
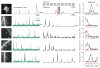
Substantial evidence at the subcellular level indicates that the spatial arrangement of synaptic inputs onto dendrites could play a significant role in cortical computations, but how synapses of functionally defined cortical networks are arranged within the dendrites of individual neurons remains unclear. Here we assessed one-dimensional spatial receptive fields of individual dendritic spines within individual layer 2/3 neuron dendrites. Spatial receptive field properties of dendritic spines were strikingly diverse, with no evidence of large-scale topographic organization. At a fine scale, organization was evident: neighboring spines separated by less than 10 μm shared similar spatial receptive field properties and exhibited a distance-dependent correlation in sensory-driven and spontaneous activity patterns. Fine-scale dendritic organization was supported by the fact that functional groups of spines defined by dimensionality reduction of receptive field properties exhibited non-random dendritic clustering. Our results demonstrate that functional synaptic clustering is a robust feature existing at a local spatial scale. VIDEO ABSTRACT.
Keywords: dendritic spine; synaptic cluster; visual cortex.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures

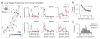



References
-
- Ascoli GA, Donohue DE, Halavi M. NeuroMorpho.Org: a central resource for neuronal morphologies. The Journal Neuroscience. 2007;27(35):9247–51. - PMC - PubMed
-
- Caliński T, Harabasz J. A dendrite method for cluster analysis. Communications in Statistics-theory and Methods. 1974;3(1):1–27.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

