17β-Estradiol Directly Lowers Mitochondrial Membrane Microviscosity and Improves Bioenergetic Function in Skeletal Muscle
- PMID: 29103922
- PMCID: PMC5762397
- DOI: 10.1016/j.cmet.2017.10.003
17β-Estradiol Directly Lowers Mitochondrial Membrane Microviscosity and Improves Bioenergetic Function in Skeletal Muscle
Abstract
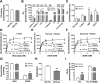
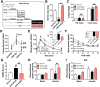
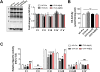
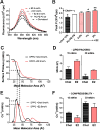
Menopause results in a progressive decline in 17β-estradiol (E2) levels, increased adiposity, decreased insulin sensitivity, and a higher risk for type 2 diabetes. Estrogen therapies can help reverse these effects, but the mechanism(s) by which E2 modulates susceptibility to metabolic disease is not well understood. In young C57BL/6N mice, short-term ovariectomy decreased-whereas E2 therapy restored-mitochondrial respiratory function, cellular redox state (GSH/GSSG), and insulin sensitivity in skeletal muscle. E2 was detected by liquid chromatography-mass spectrometry in mitochondrial membranes and varied according to whole-body E2 status independently of ERα. Loss of E2 increased mitochondrial membrane microviscosity and H2O2 emitting potential, whereas E2 administration in vivo and in vitro restored membrane E2 content, microviscosity, complex I and I + III activities, H2O2 emitting potential, and submaximal OXPHOS responsiveness. These findings demonstrate that E2 directly modulates membrane biophysical properties and bioenergetic function in mitochondria, offering a direct mechanism by which E2 status broadly influences energy homeostasis.
Keywords: estrogen; hormone replacement therapy; hydrogen peroxide; insulin resistance; membrane viscosity; menopause; mitochondria; ovariectomy.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


References
-
- Andersen OS, Koeppe RE., 2nd Bilayer thickness and membrane protein function: an energetic perspective. Annu Rev Biophys Biomol Struct. 2007;36:107–130. - PubMed
-
- Appiah D, Winters SJ, Hornung CA. Bilateral oophorectomy and the risk of incident diabetes in postmenopausal women. Diabetes Care. 2014;37:725–733. - PubMed
-
- Arias-Loza PA, Muehlfelder M, Pelzer T. Estrogen and estrogen receptors in cardiovascular oxidative stress. Pflugers Arch. 2013;465:739–746. - PubMed
-
- Bailey CJ, Ahmed-Sorour H. Role of ovarian hormones in the long-term control of glucose homeostasis. Effects of insulin secretion. Diabetologia. 1980;19:475–481. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

