Stereocontrolled Synthesis of Spiroketals: An Engine for Chemical and Biological Discovery
- PMID: 29104308
- PMCID: PMC5665374
- DOI: 10.1002/ijch.201600134
Stereocontrolled Synthesis of Spiroketals: An Engine for Chemical and Biological Discovery
Abstract
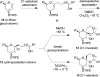
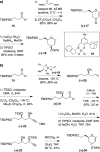
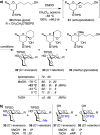
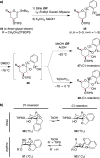
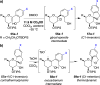
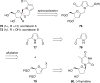
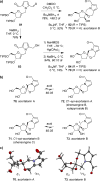
Spiroketals are key structural motifs found in diverse natural products with compelling biological activities. However, stereocontrolled synthetic access to spiroketals, independent of their inherent thermodynamic preferences, is a classical challenge in organic synthesis that has limited in-depth biological exploration of this intriguing class. Herein, we review our laboratory's efforts to advance the glycal epoxide approach to the stereocontrolled synthesis of spiroketals via kinetically controlled spirocyclization reactions. This work has provided new synthetic methodologies with applications in both diversity- and target-oriented synthesis, fundamental insights into structure and reactivity, and efficient access to spiroketal libraries and natural products for biological evaluation.
Keywords: catalysis; diversity-oriented synthesis; glycal epoxide; kinetic spiroketalization; spiro compounds.
Figures




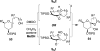



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
