PTH 1-34 Ameliorates the Osteopenia and Delayed Healing of Stabilized Tibia Fracture in Mice with Achondroplasia Resulting from Gain-Of-Function Mutation of FGFR3
- PMID: 29104492
- PMCID: PMC5666524
- DOI: 10.7150/ijbs.21258
PTH 1-34 Ameliorates the Osteopenia and Delayed Healing of Stabilized Tibia Fracture in Mice with Achondroplasia Resulting from Gain-Of-Function Mutation of FGFR3
Abstract
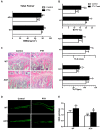
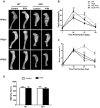
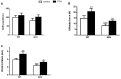
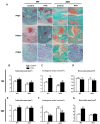
Bone fracture healing is processed through multiple stages including the cartilaginous callus formation and its transition to bony callus. FGFR3 negatively regulates chondrogenesis and enhances osteogenesis during skeleton development. We previously found in mice carrying gain-of-function mutation of FGFR3 that FGFR3 delays the healing of un-stabilized fracture that heals mainly through endochondral ossification. Since fracture is regularly treated in clinics with rigid fixation, and stabilized fracture is healed largely through intramembranous ossification, we asked whether FGFR3, a key regulator of osteogenesis, also affect the regeneration of stabilized fracture. We found that gain-of-function mutation of FGFR3 inhibits the initiation of chondrogenesis and the subsequent bone formation. We further studied whether PTH1-34 can improve the osteopenia and delayed healing of the stabilized tibia fracture in mice with achondroplasia. Fracture healing was evaluated by radiography, micro-CT, biomechanical tests, histology, and real-time polymerase chain reaction (RT-PCR) analysis. We found that PTH 1-34 can alleviate the decreased bone mass and compromised architecture in ACH mice. Histological analysis revealed that administration of PTH1-34 increased the size of both the total callus and cartilaginous callus at 14 days after the surgery in ACH mice. RT-PCR data suggested that systemic PTH1-34 accelerated the initiation of chondrogenesis and chondrocyte maturation (earlier and higher levels of expression of chondrogenesis related markers) and enhanced the osteogenic differentiation in the fracture callus in ACH mice. These results indicate that the PTH1-34 administration resulted in an enhanced callus formation during bone fracture healing in ACH mice, which is at least in part mediated by an increase of cartilaginous callus at early stage and the promotion of bone formation in bony callus. In summary, in this study we revealed that FGFR3 delays the regeneration of stabilized fracture by inhibiting both the chondrogenesis and osteogenesis, and PTH1-34 treatment can improve the dysregulated bone metabolism and delayed bone injury healing resulting from gain-of-function mutation of FGFR3.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Ornitz DM, Marie PJ. FGF signaling pathways in endochondral and intramembranous bone development and human genetic disease. Genes & development. 2002;16:1446–65. - PubMed
-
- Escobar LF, Tucker M, Bamshad M. A second family with CATSHL syndrome: Confirmatory report of another unique FGFR3 syndrome. American journal of medical genetics Part A. 2016;170:1908–11. - PubMed
-
- Valverde-Franco G, Liu H, Davidson D, Chai S, Valderrama-Carvajal H, Goltzman D. et al. Defective bone mineralization and osteopenia in young adult FGFR3-/- mice. Human molecular genetics. 2004;13:271–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

