Smart watch-based coaching with tiotropium and olodaterol ameliorates physical activity in patients with chronic obstructive pulmonary disease
- PMID: 29104624
- PMCID: PMC5658686
- DOI: 10.3892/etm.2017.5088
Smart watch-based coaching with tiotropium and olodaterol ameliorates physical activity in patients with chronic obstructive pulmonary disease
Abstract
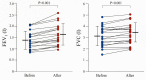
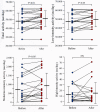
Combined therapy with tiotropium and olodaterol notably improves parameters of lung function and quality of life in patients with chronic obstructive pulmonary disease (COPD) compared to mono-components; however, its effect on physical activity is unknown. The present study evaluated whether combination therapy affects daily physical performance in patients with COPD under a smart watch-based encouragement program. This was a non-blinded clinical trial with no randomization or placebo control. A total of 20 patients with COPD were enrolled in the present study. The patients carried an accelerometer for 4 weeks; they received no therapy during the first 2 weeks but they were treated with combined tiotropium and olodaterol under a smart watch-based encouragement program for the last 2 weeks. The pulmonary function test, COPD assessment test, 6-min walk distance and parameters of physical activity were significantly improved (P<0.05) by combination therapy under smart watch-based coaching compared with values prior to treatment. To the best of our knowledge, the present study for the first time provides evidence that smart watch-based coaching in combination with tiotropium and olodaterol may improve daily physical activity in chronic obstructive pulmonary disease.
Keywords: chronic obstructive pulmonary disease; long-acting muscarinic antagonist; long-acting β2-agonist; physical activity; smart watch.
Figures


References
-
- Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Lancet Physical Activity Series Working Group: Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of burden of disease and life expectancy. Lancet. 2012;380:219–229. doi: 10.1016/S0140-6736(12)61031-9. - DOI - PMC - PubMed
-
- Benzo RP, Chang CC, Farrell MH, Kaplan R, Ries A, Martinez FJ, Wise R, Make B, Sciurba F. NETT Research Group: Physical activity, health status and risk of hospitalization in patients with severe chronic obstructive pulmonary disease. Respiration. 2010;80:10–18. doi: 10.1159/000296504. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
