A Novel Nano-approach for Targeted Inner Ear Imaging
- PMID: 29104815
- PMCID: PMC5669391
- DOI: 10.4172/2157-7439.1000456
A Novel Nano-approach for Targeted Inner Ear Imaging
Abstract
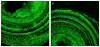
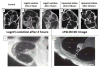
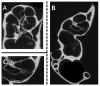
During the last decade, there have been major improvements in imaging modalities and the development of molecular imaging in general. However detailed inner ear imaging still provides very limited information to physicians. This is unsatisfactory as sensorineural hearing loss is the main cause of permanent hearing loss in adults and at least 134 genetic mutations that result in congenital hearing loss have been identified. We are still unable, in most cases where gross anatomical changes are not observed, to determine the exact cause of hearing loss at a cellular or molecular level in patients using non-invasive techniques. This limitation in inner ear diagnostic modalities is a major obstacle behind the delay in discovering treatments for many of the causes of sensorineural hearing loss. This paper initially investigated the use of targeted gold nanoparticles as contrast agents for inner ear imaging. These nanoparticles have many useful characteristics such as being easy to target and possessing minimal cytotoxicity. We were able to detect the nanoparticles diffusing in the hair cells using confocal microscopy. Regrettably, despite their many admirable characteristics, the gold nanoparticles were unable to significantly enhance CT imaging of the inner ear. Consequently, we investigated liposomal iodine as a potential solution for the unsatisfactory CT contrast obtained with the gold nanoparticles. Fortunately, significant enhancement of the micro-CT image was observed with either Lugol's solution or liposomal iodine, with Lugol's solution enabling fine inner ear structures to be detected.
Keywords: Gold nanoparticles; Inner ear imaging; Liposomal iodine; Targeted contrast agents.
Figures





References
-
- Vio MM, Holme RH. Hearing loss and tinnitus: 250 million people and a US$10 billion potential market. Drug discovery today. 2005;10:1263–1265. - PubMed
-
- Dror AA, Avraham KB. Hearing loss: mechanisms revealed by genetics and cell biology. Annu Rev Genet. 2009;43:411–437. - PubMed
-
- Joshi VM, Navlekar SK, Kishore GR, Reddy KJ, Kumar EC. CT and MR imaging of the inner ear and brain in children with congenital sensorineural hearing loss. Radiographics. 2012;32:683–698. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
