Efficacy and safety of intravenous vernakalant for the rapid conversion of recent-onset atrial fibrillation: A meta-analysis
- PMID: 29105209
- PMCID: PMC6931449
- DOI: 10.1111/anec.12508
Efficacy and safety of intravenous vernakalant for the rapid conversion of recent-onset atrial fibrillation: A meta-analysis
Abstract
Background: Atrial fibrillation is a common cardiac arrhythmia with increasing prevalence in the aging population. It is a major cause of emergency department visits worldwide. Vernakalant, a relatively new antiarrhythmic drug with selectively preferential effects on the atrial tissue is currently used in many European countries for the termination of recent-onset atrial fibrillation. Presently, the drug is still not approved by the United States Food and Drug Administration due to safety concerns. We evaluate the efficacy and safety of vernakalant for the conversion of recent-onset atrial fibrillation or atrial flutter into normal sinus rhythm (NSR).
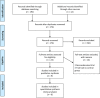
Methods: PubMed/MEDLINE (1993-2017), the Cochrane Central Register of Controlled Trials (2000-2017), and reference lists of relevant articles were searched for randomized controlled trials (RCTs) comparing vernakalant to a control drug and extracted subsequently.
Results: Nine RCTs were identified and included in the meta-analysis. Pooled analysis of events extracted for a total of 1421 patients with recent-onset atrial fibrillation showed a statistically significant increase in cardioversion within 90 minutes from drug infusion (Relative Risk [RR], 6.61; 95% Confidence Interval [CI], 2.78 - 15.71; p < .00001). In terms of adverse events, vernakalant was considered safe in comparison to control drugs (RR, 0.80; 95% CI, 0.61-1.05; p = .11).
Conclusion: Vernakalant is effective for rapid conversion of recent-onset atrial fibrillation into NSR. However, although it showed a safe profile in terms of side effects in this analysis, we are still hesitant about this conclusion and few safety issues should be addressed within specific patients' subgroups.
Keywords: atrial fibrillation/atrial arrhythmias; cardioversion; pharmacokinetics/dynamics; pharmacology.
© 2017 Wiley Periodicals, Inc.
Conflict of interest statement
The authors have no conflict of interest.
Figures






References
-
- Camm, J. (2012). Antiarrhythmic drugs for the maintenance of sinus rhythm: Risks and benefits. International journal of cardiology, 155, 362–371. - PubMed
-
- Camm, A. , Capucci, A. , Hohnloser, S. , Torp‐Pedersen, C. , Gelder, I. , Mangal, B. , & Beatch, G. . (2011). A randomized active‐controlled study comparing the efficacy and safety of vernakalant to amiodarone in recent‐onset atrial fibrillation. Journal of the American College of Cardiology, 57, 313–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

