Spinal Cord Stimulation for Treating Chronic Pain: Reviewing Preclinical and Clinical Data on Paresthesia-Free High-Frequency Therapy
- PMID: 29105244
- PMCID: PMC5766402
- DOI: 10.1111/ner.12721
Spinal Cord Stimulation for Treating Chronic Pain: Reviewing Preclinical and Clinical Data on Paresthesia-Free High-Frequency Therapy
Abstract
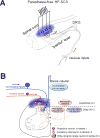
Background: Traditional spinal cord stimulation (SCS) requires that paresthesia overlaps chronic painful areas. However, the new paradigm high-frequency SCS (HF-SCS) does not rely on paresthesia.
Study design: A review of preclinical and clinical studies regarding the use of paresthesia-free HF-SCS for various chronic pain states.
Methods: We reviewed available literatures on HF-SCS, including Nevro's paresthesia-free ultra high-frequency 10 kHz therapy (HF10-SCS). Data sources included relevant literature identified through searches of PubMed, MEDLINE/OVID, and SCOPUS, and manual searches of the bibliographies of known primary and review articles.
Outcome measures: The primary goal is to describe the present developing conceptions of preclinical mechanisms of HF-SCS and to review clinical efficacy on paresthesia-free HF10-SCS for various chronic pain states.
Results: HF10-SCS offers a novel pain reduction tool without paresthesia for failed back surgery syndrome and chronic axial back pain. Preclinical findings indicate that potential mechanisms of action for paresthesia-free HF-SCS differ from those of traditional SCS.
Conclusions: To fully understand and utilize paresthesia-free HF-SCS, mechanistic study and translational research will be very important, with increasing collaboration between basic science and clinical communities to design better trials and optimize the therapy based on mechanistic findings from effective preclinical models and approaches. Future research in these vital areas may include preclinical and clinical components conducted in parallel to optimize the potential of this technology.
Keywords: Chronic pain; dorsal horn; frequency; paresthesia; spinal cord stimulation.
© 2017 International Neuromodulation Society.
Conflict of interest statement
Figures
References
-
- Shealy CN, Mortimer JT, Hagfors NR. Dorsal column electroanalgesia. J Neurosurg. 1970;32(5):560–564. - PubMed
-
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150(699):971–979. - PubMed
-
- Kumar K, Taylor RS, Jacques L, Eldabe S, Meglio M, Molet J, et al. Spinal cord stimulation versus conventional medical management for neuropathic pain: a multicentre randomised controlled trial in patients with failed back surgery syndrome. Pain. 2007;132(1–2):179–188. - PubMed
-
- Falowski S, Sharan A. A review on spinal cord stimulation. J Neurosurg Sci. 2012;56(4):287–298. - PubMed
-
- Deer TR, Mekhail N, Provenzano D, Pope J, Krames E, Leong M, et al. The appropriate use of neurostimulation of the spinal cord and peripheral nervous system for the treatment of chronic pain and ischemic diseases: the Neuromodulation Appropriateness Consensus Committee. Neuromodulation. 2014;17(6):515–550. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

