Auto-Tandem Catalysis: PdII -Catalysed Dehydrogenation/Oxidative Heck Reaction of Cyclopentane-1,3-diones
- PMID: 29105890
- PMCID: PMC5767738
- DOI: 10.1002/chem.201704442
Auto-Tandem Catalysis: PdII -Catalysed Dehydrogenation/Oxidative Heck Reaction of Cyclopentane-1,3-diones
Abstract
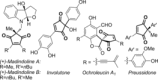
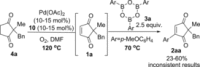
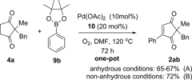
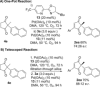
A PdII catalyst system has been used to successfully catalyse two mechanistically distinct reactions in a one-pot procedure: dehydrogenation of 2,2-disubstituted cyclopentane-1,3-diones and the subsequent oxidative Heck coupling. This auto-tandem catalytic reaction is applicable to both batch and continuous flow processes, with the latter being the first example of a tandem aerobic dehydrogenation/oxidative Heck in flow. In addition, a telescoped reaction involving enantioselective desymmetrisation of the all-C quaternary centre was successfully achieved.
Keywords: aerobic oxidation; auto-tandem catalysis; one-pot synthesis; oxidative Heck; palladium.
© 2017 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures



Similar articles
-
A versatile approach to flavones via a one-pot Pd(II)-catalyzed dehydrogenation/oxidative boron-Heck coupling sequence of chromanones.Org Biomol Chem. 2016 Jan 14;14(2):777-784. doi: 10.1039/c5ob01911g. Org Biomol Chem. 2016. PMID: 26592753
-
Oxidative Heck desymmetrisation of 2,2-disubstituted cyclopentene-1,3-diones.Chem Commun (Camb). 2015 Mar 7;51(19):4089-92. doi: 10.1039/c5cc00407a. Chem Commun (Camb). 2015. PMID: 25665602
-
Tandem-type Pd(II)-catalyzed oxidative Heck reaction/intramolecular C-H amidation sequence: a novel route to 4-aryl-2-quinolinones.Chem Commun (Camb). 2012 May 7;48(36):4332-4. doi: 10.1039/c2cc30600j. Epub 2012 Mar 23. Chem Commun (Camb). 2012. PMID: 22441307
-
Enantioselective Dicarbofunctionalization of Unactivated Alkenes by Palladium-Catalyzed Tandem Heck/Suzuki Coupling Reaction.Angew Chem Int Ed Engl. 2019 Oct 7;58(41):14653-14659. doi: 10.1002/anie.201907840. Epub 2019 Sep 5. Angew Chem Int Ed Engl. 2019. PMID: 31420928 Review.
-
Synthesis of heterocyclic compounds through palladium-catalyzed C-H cyclization processes.Chem Pharm Bull (Tokyo). 2013;61(10):987-96. doi: 10.1248/cpb.c13-00420. Chem Pharm Bull (Tokyo). 2013. PMID: 24088691 Review.
Cited by
-
Ligand-free Pd-catalyzed highly selective arylation of activated and unactivated alkenes via oxidative and reductive heck coupling.RSC Adv. 2024 Feb 21;14(10):6470-6475. doi: 10.1039/d3ra08186a. eCollection 2024 Feb 21. RSC Adv. 2024. PMID: 38390499 Free PMC article.
-
Designing a Redox Noninnocent Phenalenyl-Based Copper(II) Complex: An Autotandem Catalyst for the Selective Oxidation of Polycyclic Aromatic Hydrocarbons (PAHs).ACS Omega. 2022 Mar 1;7(10):8789-8797. doi: 10.1021/acsomega.1c07051. eCollection 2022 Mar 15. ACS Omega. 2022. PMID: 35309439 Free PMC article.
References
-
- Review: Sevcikova Z., Pour M., Novak D., Ulrichova J., Vacek J., Mini Rev. Med. Chem. 2014, 14, 322–331. - PubMed
-
- None
-
- Hirose T., Sunazuka T., Shirahata T., Yamamoto D., Harigaya Y., Kuwajima I., Omura S., Org. Lett. 2002, 4, 501–503; - PubMed
-
- Hirose T., Sunazuka T., Yamamoto D., Kaji E., Omura S., Tetrahedron Lett. 2006, 47, 6761–6764;
-
- Hosokawa S., Sekiguchi K., Hayase K., Hirukawa Y., Kobayashi S., Tetrahedron Lett. 2000, 41, 6435–6439;
LinkOut - more resources
Full Text Sources
Other Literature Sources

