Genetic and neuronal regulation of sleep by neuropeptide VF
- PMID: 29106375
- PMCID: PMC5705210
- DOI: 10.7554/eLife.25727
Genetic and neuronal regulation of sleep by neuropeptide VF
Abstract
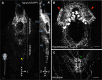
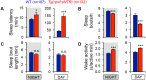
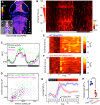
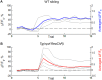
Sleep is an essential and phylogenetically conserved behavioral state, but it remains unclear to what extent genes identified in invertebrates also regulate vertebrate sleep. RFamide-related neuropeptides have been shown to promote invertebrate sleep, and here we report that the vertebrate hypothalamic RFamide neuropeptide VF (NPVF) regulates sleep in the zebrafish, a diurnal vertebrate. We found that NPVF signaling and npvf-expressing neurons are both necessary and sufficient to promote sleep, that mature peptides derived from the NPVF preproprotein promote sleep in a synergistic manner, and that stimulation of npvf-expressing neurons induces neuronal activity levels consistent with normal sleep. These results identify NPVF signaling and npvf-expressing neurons as a novel vertebrate sleep-promoting system and suggest that RFamide neuropeptides participate in an ancient and central aspect of sleep control.
Keywords: RFamide neuropeptide; behavior; genetics; hypothalamus; neuropeptide VF; neuroscience; sleep; zebrafish.
Conflict of interest statement
No competing interests declared.
Figures


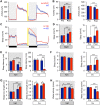



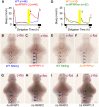
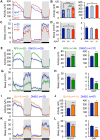
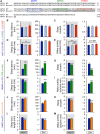






Similar articles
-
Neuropeptide VF neurons promote sleep via the serotonergic raphe.Elife. 2020 Dec 18;9:e54491. doi: 10.7554/eLife.54491. Elife. 2020. PMID: 33337320 Free PMC article.
-
The hypothalamic NPVF circuit modulates ventral raphe activity during nociception.Sci Rep. 2017 Jan 31;7:41528. doi: 10.1038/srep41528. Sci Rep. 2017. PMID: 28139691 Free PMC article.
-
Neuropeptide VF-associated satiety involves mu and kappa but not delta subtypes of opioid receptors in chicks.Neurosci Lett. 2009 May 22;455(3):195-8. doi: 10.1016/j.neulet.2009.03.029. Epub 2009 Mar 14. Neurosci Lett. 2009. PMID: 19429120
-
The distribution of Neuropeptide FF and Neuropeptide VF in central and peripheral tissues and their role in energy homeostasis control.Neuropeptides. 2021 Dec;90:102198. doi: 10.1016/j.npep.2021.102198. Epub 2021 Sep 9. Neuropeptides. 2021. PMID: 34534716 Review.
-
Structure and functions of the novel hypothalamic RFamide neuropeptides R-RFa and 26RFa in vertebrates.Peptides. 2006 May;27(5):1110-20. doi: 10.1016/j.peptides.2005.06.035. Epub 2006 Mar 6. Peptides. 2006. PMID: 16517014 Review.
Cited by
-
Evolutionarily Conserved Regulation of Sleep by the Protein Translational Regulator PERK.Curr Biol. 2020 May 4;30(9):1639-1648.e3. doi: 10.1016/j.cub.2020.02.030. Epub 2020 Mar 12. Curr Biol. 2020. PMID: 32169212 Free PMC article.
-
Synaptic dysfunction connects autism spectrum disorder and sleep disturbances: A perspective from studies in model organisms.Sleep Med Rev. 2022 Apr;62:101595. doi: 10.1016/j.smrv.2022.101595. Epub 2022 Jan 25. Sleep Med Rev. 2022. PMID: 35158305 Free PMC article. Review.
-
Validation of Candidate Sleep Disorder Risk Genes Using Zebrafish.Front Mol Neurosci. 2022 Apr 7;15:873520. doi: 10.3389/fnmol.2022.873520. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35465097 Free PMC article. Review.
-
Recent advances in sleep genetics.Curr Opin Neurobiol. 2021 Aug;69:19-24. doi: 10.1016/j.conb.2020.11.012. Epub 2020 Dec 25. Curr Opin Neurobiol. 2021. PMID: 33360546 Free PMC article. Review.
-
Neuropeptides and Behaviors: How Small Peptides Regulate Nervous System Function and Behavioral Outputs.Front Mol Neurosci. 2021 Dec 2;14:786471. doi: 10.3389/fnmol.2021.786471. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34924955 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

