Angiopoietin-1 is required for Schlemm's canal development in mice and humans
- PMID: 29106382
- PMCID: PMC5707160
- DOI: 10.1172/JCI95545
Angiopoietin-1 is required for Schlemm's canal development in mice and humans
Abstract
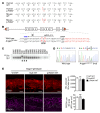
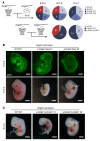
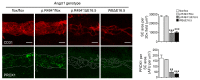
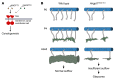
Primary congenital glaucoma (PCG) is a leading cause of blindness in children worldwide and is caused by developmental defects in 2 aqueous humor outflow structures, Schlemm's canal (SC) and the trabecular meshwork. We previously identified loss-of-function mutations in the angiopoietin (ANGPT) receptor TEK in families with PCG and showed that ANGPT/TEK signaling is essential for SC development. Here, we describe roles for the major ANGPT ligands in the development of the aqueous outflow pathway. We determined that ANGPT1 is essential for SC development, and that Angpt1-knockout mice form a severely hypomorphic canal with elevated intraocular pressure. By contrast, ANGPT2 was dispensable, although mice deficient in both Angpt1 and Angpt2 completely lacked SC, indicating that ANGPT2 compensates for the loss of ANGPT1. In addition, we identified 3 human subjects with rare ANGPT1 variants within an international cohort of 284 PCG patients. Loss of function in 2 of the 3 patient alleles was observed by functional analysis of ANGPT1 variants in a combined in silico, in vitro, and in vivo approach, supporting a causative role for ANGPT1 in disease. By linking ANGPT1 with PCG, these results highlight the importance of ANGPT/TEK signaling in glaucoma pathogenesis and identify a candidate target for therapeutic development.
Keywords: Mouse models; Ophthalmology; Vascular Biology; endothelial cells.
Conflict of interest statement
Figures
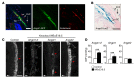
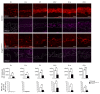

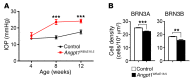


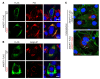
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

