Selective neuronal lapses precede human cognitive lapses following sleep deprivation
- PMID: 29106402
- PMCID: PMC5720899
- DOI: 10.1038/nm.4433
Selective neuronal lapses precede human cognitive lapses following sleep deprivation
Abstract
Sleep deprivation is a major source of morbidity with widespread health effects, including increased risk of hypertension, diabetes, obesity, heart attack, and stroke. Moreover, sleep deprivation brings about vehicle accidents and medical errors and is therefore an urgent topic of investigation. During sleep deprivation, homeostatic and circadian processes interact to build up sleep pressure, which results in slow behavioral performance (cognitive lapses) typically attributed to attentional thalamic and frontoparietal circuits, but the underlying mechanisms remain unclear. Recently, through study of electroencephalograms (EEGs) in humans and local field potentials (LFPs) in nonhuman primates and rodents it was found that, during sleep deprivation, regional 'sleep-like' slow and theta (slow/theta) waves co-occur with impaired behavioral performance during wakefulness. Here we used intracranial electrodes to record single-neuron activities and LFPs in human neurosurgical patients performing a face/nonface categorization psychomotor vigilance task (PVT) over multiple experimental sessions, including a session after full-night sleep deprivation. We find that, just before cognitive lapses, the selective spiking responses of individual neurons in the medial temporal lobe (MTL) are attenuated, delayed, and lengthened. These 'neuronal lapses' are evident on a trial-by-trial basis when comparing the slowest behavioral PVT reaction times to the fastest. Furthermore, during cognitive lapses, LFPs exhibit a relative local increase in slow/theta activity that is correlated with degraded single-neuron responses and with baseline theta activity. Our results show that cognitive lapses involve local state-dependent changes in neuronal activity already present in the MTL.
Conflict of interest statement
Figures
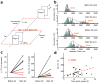
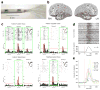
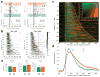

References
-
- Colten HR, Altevogt BM, editors. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. Washington (DC): 2006. - PubMed
-
- Lyznicki JM, Doege TC, Davis RM, Williams MA. Sleepiness, driving, and motor vehicle crashes. Council on Scientific Affairs, American Medical Association. Jama. 1998;279:1908–1913. - PubMed
-
- Borbely AA. A two process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. - PubMed

