Vaccination With a Latch Peptide Provides Serotype-Independent Protection Against Group B Streptococcus Infection in Mice
- PMID: 29106586
- PMCID: PMC5854025
- DOI: 10.1093/infdis/jix565
Vaccination With a Latch Peptide Provides Serotype-Independent Protection Against Group B Streptococcus Infection in Mice
Abstract
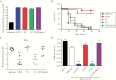
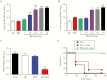
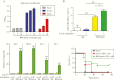
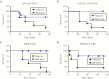
Streptococcus agalactiae (group B streptococcus [GBS]) is a leading cause of invasive diseases in neonates and severe infections in elderly individuals. GBS serine-rich repeat glycoprotein 1 (Srr1) acts as a critical virulence factor by facilitating GBS invasion into the central nervous system through interaction with the fibrinogen Aα chain. This study revealed that srr1 is highly conserved, with 86.7% of GBS clinical isolates expressing the protein. Vaccination of mice with different Srr1 truncated peptides revealed that only Srr1 truncates containing the latch domain protected against GBS meningitis. Furthermore, the latch peptide alone was immunogenic and elicited protective antibodies, which efficiently enhanced antibody-mediated opsonophagocytic killing of GBS by HL60 cells and provided heterogeneous protection against 4 different GBS serogroups. Taken together, these findings indicated that the latch domain of Srr1 may constitute an effective peptide vaccine candidate for GBS.
Keywords: Streptococcus agalactiae; latch; serine-rich repeat; vaccine.
© The Author(s) 2017. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures

References
-
- Ballard MS, Schønheyder HC, Knudsen JD et al. ; International Bacteremia Surveillance Collaborative. The changing epidemiology of group B streptococcus bloodstream infection: a multi-national population-based assessment. Infect Dis (Lond) 2016; 48:386–91. - PubMed
-
- Shibayama A, Yoshizaki T, Tamaki M, Goto M, Takahashi T. Pyogenic Sternoclavicular Arthritis Caused by Streptococcus agalactiae in an Elderly Adult with Diabetes Mellitus. J Am Geriatr Soc 2016; 64:1376–7. - PubMed
-
- Johnson FM, Garcia A. Sepsis with group B streptococci (Streptococcus agalactiae) secondary to urinary tract infection in an adult male. J Am Geriatr Soc 1999; 47:629–30. - PubMed
-
- Berardi A, Rossi C, Bacchi Reggiani ML et al. An area-based study on intrapartum antibiotic prophylaxis for preventing group B streptococcus early-onset disease: advances and limitations. J Matern Fetal Neonatal Med 2017; 30:1739–44. - PubMed
-
- Creti R, Imperi M, Berardi A et al. ; Italian Neonatal GBS Infections Working Group. Neonatal group B streptococcus infections: prevention strategies, clinical and microbiologic characteristics in 7 years of surveillance. Pediatr Infect Dis J 2017; 36:256–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

