Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143
- PMID: 29106665
- PMCID: PMC5892140
- DOI: 10.1093/neuonc/nox208
Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143
Abstract
Background: Immunotherapies have demonstrated efficacy across a diverse set of tumors supporting further evaluation in glioblastoma. The objective of this study was to evaluate the safety/tolerability and describe immune-mediated effects of nivolumab ± ipilimumab in patients with recurrent glioblastoma. Exploratory efficacy outcomes are also reported.
Methods: Patients were randomized to receive nivolumab 3 mg/kg every 2 weeks (Q2W; NIVO3) or nivolumab 1 mg/kg + ipilimumab 3 mg/kg every 3 weeks (Q3W) for 4 doses, then nivolumab 3 mg/kg Q2W (NIVO1+IPI3). An alternative regimen of nivolumab 3 mg/kg + ipilimumab 1 mg/kg Q3W for 4 doses, then nivolumab 3 mg/kg Q2W (NIVO3+IPI1) was investigated in a nonrandomized arm.
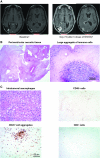
Results: Forty patients were enrolled (NIVO3, n = 10; NIVO1+IPI3, n = 10; NIVO3+IPI1, n = 20). The most common treatment-related adverse events (AEs) were fatigue (NIVO3, 30%; NIVO1+IPI3, 80%; NIVO3+IPI1, 55%) and diarrhea (10%, 70%, 30%, respectively). AEs leading to discontinuation occurred in 10% (NIVO3), 30% (NIVO1+IPI3), and 20% (NIVO3+IPI1) of patients. Three patients achieved a partial response (NIVO3, n = 1; NIVO3+IPI1, n = 2) and 8 had stable disease for ≥12 weeks (NIVO3, n = 2; NIVO1+IPI3, n = 2; NIVO3+IPI1, n = 4 [Response Assessment in Neuro-Oncology criteria]). Most patients (68%) had tumor-cell programmed death ligand-1 expression ≥1%. Immune-mediated effects mimicking radiographic progression occurred in 2 patients.
Conclusions: Nivolumab monotherapy was better tolerated than nivolumab + ipilimumab; the tolerability of the combination was influenced by ipilimumab dose. These safety and exploratory findings merit further investigation of immunotherapies in glioblastoma.
Figures

References
-
- Stupp R, Hegi ME, Mason WP et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. - PubMed
-
- National Comprehensive Cancer Network. 2017. NCCN Clinical Practice Guidelines in Oncology. Central Nervous System Cancers. Version I 2017. Available at https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed September 15, 2017.
-
- Campos B, Olsen LR, Urup T, Poulsen HS. A comprehensive profile of recurrent glioblastoma. Oncogene. 2016;35(45):5819–5825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

