Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction
- PMID: 29106912
- PMCID: PMC5846093
- DOI: 10.1016/j.trsl.2017.10.001
Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction
Abstract
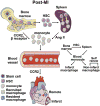
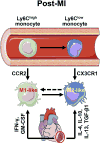
Macrophages play critical roles in homeostatic maintenance of the myocardium under normal conditions and in tissue repair after injury. In the steady-state heart, resident cardiac macrophages remove senescent and dying cells and facilitate electrical conduction. In the aging heart, the shift in macrophage phenotype to a proinflammatory subtype leads to inflammaging. Following myocardial infarction (MI), macrophages recruited to the infarct produce both proinflammatory and anti-inflammatory mediators (cytokines, chemokines, matrix metalloproteinases, and growth factors), phagocytize dead cells, and promote angiogenesis and scar formation. These diverse properties are attributed to distinct macrophage subtypes and polarization status. Infarct macrophages exhibit a proinflammatory M1 phenotype early and become polarized toward an anti-inflammatory M2 phenotype later post-MI. Although this classification system is oversimplified and needs to be refined to accommodate the multiple different macrophage subtypes that have been recently identified, general concepts on macrophage roles are independent of subtype classification. This review summarizes current knowledge about cardiac macrophage origins, roles, and phenotypes in the steady state, with aging, and after MI, as well as highlights outstanding areas of investigation.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have read the journal authorship agreement and policy on disclosure of potential conflicts of interest and have nothing to disclose.
Figures

References
-
- Gordon S. The macrophage: past, present and future. Eur J Immunol. 2007;37:S9–17. - PubMed
-
- Tamoutounour S, Guilliams M, Montanana Sanchis F, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–38. - PubMed
-
- Zigmond E, Varol C, Farache J, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

