Therapeutic targeting of PP2A
- PMID: 29107183
- PMCID: PMC5927617
- DOI: 10.1016/j.biocel.2017.10.008
Therapeutic targeting of PP2A
Abstract
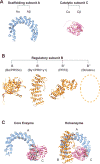
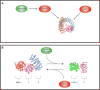
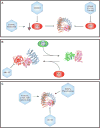
Protein phosphatase 2A (PP2A) is a major serine/threonine phosphatase that regulates many cellular processes. Given the central role of PP2A in regulating diverse biological functions and its dysregulation in many diseases, including cancer, PP2A directed therapeutics have become of great interest. The main approaches leveraged thus far can be categorized as follows: 1) inhibiting endogenous inhibitors of PP2A, 2) targeted disruption of post translational modifications on PP2A subunits, or 3) direct targeting of PP2A. Additional insight into the structural, molecular, and biological framework driving the efficacy of these therapeutic strategies will provide a foundation for the refinement and development of novel and clinically tractable PP2A targeted therapies.
Keywords: ABL-127; Bortezomib; CIP2A; Cancer; Celastrol; Cell signaling; Drug development; Erlotinib; FTY720; LB-100; LCMT-1; OP449; PME-1; PP2A; Phenothiazines; Protein phosphatase 2A; Protein phosphatases; SET; SMAPs; Tumor suppressors.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
References
-
- Adachi Y, Pavlakis GN, Copeland TD. Identification and Characterization of SET, a Nuclear Phosphoprotein Encoded by the Translocation Break Point in Acute Undifferentiated Leukemia*. J BIOUX~ICAL Chem. 1994;269:2258–2262. - PubMed
-
- Agarwal A, MacKenzie R, Oddo J, Vitek MP, Christensen DJ, Druker BJ. A Novel SET Antagonist (OP449) Is Cytotoxic to CML Cells, Including the Highly-Resistant BCR-ABLT315I Mutant, and Demonstrates Enhanced Efficacy in Combination with ABL Tyrosine Kinase Inhibitors. Blood. 2011;118
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

