The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice
- PMID: 29107282
- PMCID: PMC5681270
- DOI: 10.1016/j.molmet.2017.08.010
The autonomic nervous system and cardiac GLP-1 receptors control heart rate in mice
Abstract
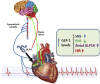
Objectives: Glucagon-like peptide-1 (GLP-1) is secreted from enteroendocrine cells and exerts a broad number of metabolic actions through activation of a single GLP-1 receptor (GLP-1R). The cardiovascular actions of GLP-1 have garnered increasing attention as GLP-1R agonists are used to treat human subjects with diabetes and obesity that may be at increased risk for development of heart disease. Here we studied mechanisms linking GLP-1R activation to control of heart rate (HR) in mice.
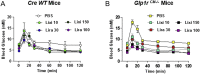
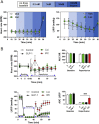
Methods: The actions of GLP-1R agonists were examined on the control of HR in wild type mice (WT) and in mice with cardiomyocyte-selective disruption of the GLP-1R (Glp1rCM-/-). Complimentary studies examined the effects of GLP-1R agonists in mice co-administered propranolol or atropine. The direct effects of GLP-1R agonism on HR and ventricular developed pressure were examined in isolated perfused mouse hearts ex vivo, and atrial depolarization was quantified in mouse hearts following direct application of liraglutide to perfused atrial preparations ex vivo.
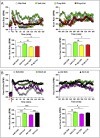
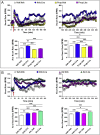
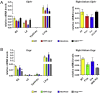
Results: Doses of liraglutide and lixisenatide that were equipotent for acute glucose control rapidly increased HR in WT and Glp1rCM-/- mice in vivo. The actions of liraglutide to increase HR were more sustained relative to lixisenatide, and diminished in Glp1rCM-/- mice. The acute chronotropic actions of GLP-1R agonists were attenuated by propranolol but not atropine. Neither native GLP-1 nor lixisenatide increased HR or developed pressure in perfused hearts ex vivo. Moreover, liraglutide had no direct effect on sinoatrial node firing rate in mouse atrial preparations ex vivo. Despite co-localization of HCN4 and GLP-1R in primate hearts, HCN4-directed Cre expression did not attenuate levels of Glp1r mRNA transcripts, but did reduce atrial Gcgr expression in the mouse heart.
Conclusions: GLP-1R agonists increase HR through multiple mechanisms, including regulation of autonomic nervous system function, and activation of the atrial GLP-1R. Surprisingly, the isolated atrial GLP-1R does not transduce a direct chronotropic effect following exposure to GLP-1R agonists in the intact heart, or isolated atrium, ex vivo. Hence, cardiac GLP-1R circuits controlling HR require neural inputs and do not function in a heart-autonomous manner.
Keywords: Autonomic nervous system; Cardiac; Cardiovascular disease; Diabetes; GLP-1; Heart rate.
Copyright © 2017 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures


References
-
- Drucker D.J. The cardiovascular biology of glucagon-like Peptide-1. Cell Metabolism. 2016;24(1):15–30. - PubMed
-
- Stamler J., Vaccaro O., Neaton J.D., Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434–444. - PubMed
-
- Zinman B., Wanner C., Lachin J.M., Fitchett D., Bluhmki E., Hantel S. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. New England Journal of Medicine. 2015;373(22):2117–2128. - PubMed
-
- Barragan J.M., Rodriguez R.E., Blazquez E. Changes in arterial blood pressure and heart rate induced by glucagon-like peptide-1-(7-36 amide) in rats. American Journal of Physiology. 1994;266:E459–E466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

