Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury
- PMID: 29107287
- PMCID: PMC5681240
- DOI: 10.1016/j.molmet.2017.08.004
Fibroblast growth factor 21 (FGF21) is robustly induced by ethanol and has a protective role in ethanol associated liver injury
Abstract
Objective: Excess ethanol consumption has serious pathologic consequences. In humans, repeated episodes of binge drinking can lead to liver damage and have adverse effects on other organs such as pancreas and brain. Long term chronic consumption of ethanol can also result in progressive alcoholic liver disease and cirrhosis. Fibroblast growth factor 21 (FGF21) is a metabolic regulator with multiple physiologic functions. FGF21 is a novel biomarker for non-alcoholic fatty liver disease (NAFLD) in humans and limits hepatotoxicity in mice. Therefore, we explored the possibility that FGF21 plays a role in response to ethanol consumption in both humans and mice.
Methods: We used a binge drinking paradigm in humans to examine the effect of acute ethanol consumption on circulating FGF21. We adapted this paradigm to evaluate the acute response to ethanol in mice. We then examined the role of FGF21 on liver pathology in two models of chronic ethanol consumption in both wild type (WT) mice and mice lacking FGF21 (FGF21-KO).
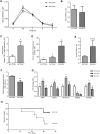
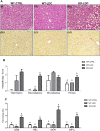
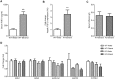
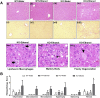
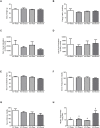
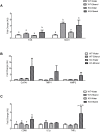
Results: Acute ethanol consumption resulted in a robust induction of serum FGF21 after 6 h in both humans and mice. Serum ethanol peaked at 1 h in both species and was cleared by 6 h. Ethanol clearance was the same in WT and FGF21-KO mice, indicating that FGF21 does not play a major role in ethanol metabolism in a binge paradigm. When FGF21-KO mice were fed the Lieber-DeCarli diet, a high fat diet supplemented with ethanol, a higher mortality was observed compared to WT mice after 16 days on the diet. When FGF21-KO mice consumed 30% ethanol in drinking water, along with a normal chow diet, there was no mortality observed even after 16 weeks, but the FGF21-KO mice had significant liver pathology compared to WT mice.
Conclusions: Acute or binge ethanol consumption significantly increases circulating FGF21 levels in both humans and mice. However, FGF21 does not play a role in acute ethanol clearance. In contrast, chronic ethanol consumption in the absence of FGF21 is associated with significant liver pathology alone or in combination with excess mortality, depending on the type of diet consumed with ethanol. This suggests that FGF21 protects against long term ethanol induced hepatic damage and may attenuate progression of alcoholic liver disease. Further study is required to assess the therapeutic potential of FGF21 in the treatment of alcoholic liver disease.
Keywords: Alcoholic liver disease; Binge ethanol consumption; Chronic ethanol consumption; FGF21.
Copyright © 2017 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures



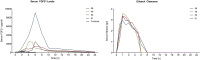
References
-
- Fisher F.M., Maratos-Flier E. Understanding the physiology of FGF21. Annual Review of Physiology. 2016;78:223–241. - PubMed
-
- Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5:426–437. - PubMed
-
- Inagaki T., Dutchak P., Zhao G., Ding X., Gautron L., Parameswara V. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metabolism. 2007;5:415–425. - PubMed
-
- Galman C., Lundasen T., Kharitonenkov A., Bina H.A., Eriksson M., Hafstrom I. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metabolism. 2008;8:169–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

